4D-STEM study of silk fibroin microstructure
- Abstract number
- 190
- Presentation Form
- Poster & Flash Talk
- DOI
- 10.22443/rms.mmc2023.190
- Corresponding Email
- [email protected]
- Session
- Poster Session One
- Authors
- Mr. Bing Han (1), Dr. Alexander Eggeman (1), Dr. Chinnawich Phamornnak (1), Dr. Jonny Blaker (1), Prof. Sarah Cartmell (1)
- Affiliations
-
1. University of Manchester
- Keywords
4D-STEM, cryogenic, low-dose, biomaterials, silk fibroin
- Abstract text
Worm silk fibroin has been widely studied in the field of nerve repairing owing to a number of favourable properties such as high tensile strength, biocompatibility, and non-toxicity[1]. Compared with conventional nerve repairing approaches such as nerve grafting, silk fibroin is biodegradable and can provide proper electrical environment (when properly doped) that stimulates the nerve growth [2] while reducing their current limitations such as tissue availability, secondary deformities, and size differences[3].
However, the study of the silk fibroin microstructure through TEM has been limited due to the energy sensitivity of the material. Organic materials, including silk fibroin, are energy sensitive because they lack sufficient delocalized electrons to repair ionization damage or remove local heat build-up[4]. This makes them vulnerable to change or damage as a result of energy deposition from the incident electron beam. The recent advancement in low-dose techniques has made direct observation of silk fibroin possible, including the use of an ultra-fast direct electron detector with a cryogenic holder (temperature ~-170℃) that allows for extremely low electron doses and improves the stability of the silk.
The typical method of preparing silk samples for tissue engineering is electrospinning[5]. Experiments were carried out on silk fibroin samples produced through general electrospinning and conducted using a Talos F200X TEM equipped with a Merlin diffraction camera and a Gatan ‘Elsa’ cryogenic holder.
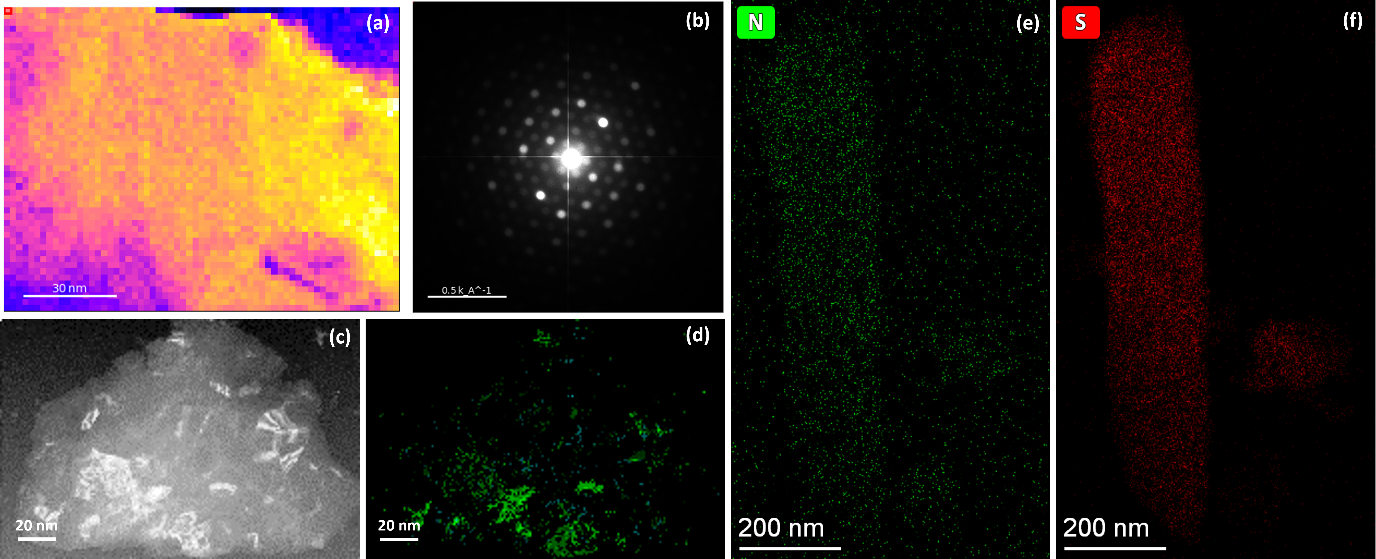
Figure 1. Virtual bright field (VBF) image of direct electrospun worm silk fibroin (a) with summed diffraction pattern (b). VBF image of PEDOT: PSS doped worm silk fibroin (c) and indexed crystal mapping image (d). Nitrogen (e) and sulphur (f) element mapping obtained with cryogenic holder under ~-170℃.
The process of making a silk fibroin sample began with an electrospinning method, followed by treatment with ethanol to re-crystallize the silk and make it non-sticky. The treated silk was then analysed using 4D-STEM (Figure 1a) under low-dose conditions to determine its crystal structure. The summed diffraction pattern in Figure 1b showed that the pristine silk followed an orthorhombic polytype commonly referred to as silk II, which is distinct from the monoclinic structure (silk I). After adding the conductive polymer PEFOT: PSS, the crystal structure of the doped silk was also analysed using 4D-STEM (Figure 1c), and the phase mapping results (Figure 1d) showed that about 92% of the diffraction patterns were consistent with the orthorhombic silk II structure. Furthermore, the size of the individual crystalline areas was larger compared to the pristine silk (Figure 1a), suggesting that the processing stages to produce the conductive polymer may have increased the crystallinity of the silk.
Additional studies were performed using XPS to explore the difference in surface chemistry between the pristine and doped silk fibroin, the nitrogen peak was found to be shifted after adding the conductive dopants (PEDOT: PSS), with a complementary change in the sulphur peaks indicative of the formation of new bonds with nitrogen. Silk II can be formed from chains of either parallel or anti-parallel amino acid strands, with the anti-parallel strand being incompletely hydrogen bonded. The incomplete hydrogen bonding provides a lot of unsaturated N-H functionality on the material and offers sites for reaction with sulphur from the PEDOT: PSS. This suggests that the potential S-N bonds could be the reaction site of the PEDOT: PSS doping process and that the conductive polymers were doped onto the silk fibroin.
Finally, the doped silk fibroin was stabilized using a cryogenic holder and EDX mapping was performed (Figure 1e and f). The results indicate that the EDX signal is strong enough and the distributions of nitrogen (Figure 1e) and sulphur (Figure 1f) are largely overlapping along the silk, supporting the hypothesis that S-N bonds may have formed during the doping process. It is possible that the formation of S-N bonds is also influenced by the crystalline regions, and this will be the focus of future experiments.
- References
- Phamornnak, C., Han, B., Spencer, B. F., Ashton, M. D., Blanford, C. F., Hardy, J. G., ... & Cartmell, S. H. (2022). Instructive electroactive electrospun silk fibroin-based biomaterials for peripheral nerve tissue engineering. Biomaterials Advances, 141, 213094.
- Wilson D H, Jagadeesh P. Experimental regeneration in peripheral nerves and the spinal cord in laboratory animals exposed to a pulsed electromagnetic field[J]. Spinal Cord, 1976, 14(1): 12-20.
- Evans G R D. Peripheral nerve injury: a review and approach to tissue engineered constructs[J]. The Anatomical Record: An Official Publication of the American Association of Anatomists, 2001, 263(4): 396-404.
- S. Inagaki, N. Goto, and K. Yoshikawa. Antibonding delocalization: Geminal Interaction of σ-bonds and Angle Strain. J. Am. Chem. Soc. 1991, 113, 7144-7146.
- Balint, Richard, Nigel J. Cassidy, and Sarah H. Cartmell. "Conductive polymers: Towards a smart biomaterial for tissue engineering." Acta biomaterialia 10.6 (2014): 2341-2353.