Sample preparation for cryoTEM - cryoLiftOut using a freezable microgripper
- Abstract number
- 7
- Presentation Form
- Poster & Flash Talk
- DOI
- 10.22443/rms.mmc2023.7
- Corresponding Email
- [email protected]
- Session
- FIB Applications & EM Sample Prep Techniques in Biological Sciences
- Authors
- Dr. Andrew Jonathan Smith (1), Andreas Rummel (1), Dr. Stephan Kleindiek (1)
- Affiliations
-
1. Kleindiek Nanotechnik
- Keywords
cryoET, cryoTEM, cryoFIB, FIB, liftout, TEM sample prep, FIBSEM, FIB, SEM
- Abstract text
Recent advances cryo electron microscopy (cryoEM) workflows are advancing research opportunities in life science and other fields. In material science, cooling samples halts aging processes yielding insights into phase transitions while also enabling new applications such as cryo Atom Probe Tomography (cryoAPT). Turing to life sciences, the most prominent application is cryo Electron Tomography (cryoET), which yields unprecedented structural information of pristine biological specimen.
Small samples (i.e. single cells) can be plunge frozen while preserving their integrity. These can be grown on-grid and processed for TEM investigation without additional steps. When dealing with larger tissue samples, however, High Pressure Freezing (HPF) is required yielding a 'puck' of frozen tissue from which a thin slice needs to be extracted. This is done by manufacturing the slice in cryo Focussed Ion Beam microscope (cryoFIB) by milling away material until the desired slice remains. The slice is then extracted from the bulk and moved to a suitable carrier (grid) for further thinning and investigation.
In conventional (room temperature) FIB, the slice is attached to a needle using beam induced deposition of material from precursor gases injected into the microscope's chamber. This process is slow, error-prone, and does not work under cryo conditions. In this case, injected material is used to freeze the lamella to the needle, coating everything in frozen layer of whatever material was injected in the process. In addition to poor reliability, this approach necessitates frequent needle exchanges as the slice needs to be cut off of the needle after every sample transfer.
Using a microgripper specifically designed for handling FIB-milled TEM specimen is an established technique that has been in use for many years [1].
This work will describe the challenges faced when redesigning such a microgripper for use under cryogenic conditions as well as the workflow development for transferring frozen lamellae from bulk samples to TEM grids for cryo Electron Tomography [2].
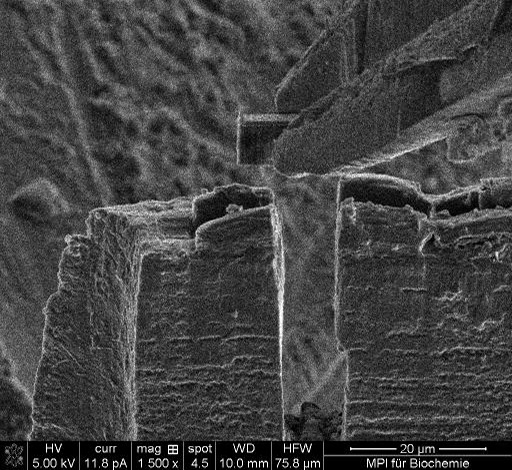
Finally, the established procedure for cryoTEM sample preparation will be demonstrated: This procedure entails grabbing the lamella with the gripper prior to making the final cut to release it from the bulk sample (Fig. 1).
Then the actual lift out is executed. Subsequently, the microscope's sample stage is used to position the grid at the point of beam coincidence - just underneath the lifted lamella and the lamella is placed on the grid (Fig. 2).
Finally, the gripper can be retracted and the next preparation cycle can commence.
- References
[1] Rummel et al., Prakt. Metallogr. 46 (2009) 5
[2] Schaffer et al. Nature Methods 16 (2019) 757–762