Ultramicrotomy reveals biotechnological synthesis conditions to control the size and location of microbially supported Pd nanoparticles
- Abstract number
- 184
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2023.184
- Corresponding Email
- [email protected]
- Session
- Poster Session Two
- Authors
- Christopher Egan Morriss (2), Casey Cheung (2), Nigel Powell (1), Sarah Haigh (2), Jonathan Lloyd (2)
- Affiliations
-
1. Johnson Matthey Technology Centre
2. University of Manchester
- Keywords
Pd nanoparticles, ultramicrotomy, TEM, bionanomaterials, catalysis
- Abstract text
Summary
Ultramicrotomy of Pd nanoparticles supported on microbial cells (bio-Pd) enabled imaging of the location of bio-Pd within the cell structure under different biosynthesis conditions. In addition, thin and uniform samples allowed for improved imaging over drop cast samples of whole cells. This study reveals the changes in location and size distribution of bio-Pd nanoparticles when supplied with different electron donors under different Pd loadings, which allows control over the catalytic properties of bio-Pd.
Introduction
The biosynthesis of metal nanoparticles supported on microbial cells has attracted recent interest as an economical, scalable, and green biotechnology [1]. Bio-Pd nanoparticles are active as heterogeneous catalysts for industrially important reactions such as hydrogenation, and C-C coupling such as Heck and Suzuki reactions [2]. Bio-Pd is synthesised via enzymatic reduction of Pd(II) to Pd(0) at ambient temperature, and requires only microbial cells, a Pd(II) source, inexpensive buffer solutions, and an electron donor. However, the effect of these synthesis conditions on the properties of bio-Pd nanoparticles is not well understood. Different electron donors cause different enzymatic reduction pathways to be used by the cells, leading to metal deposition occurring at different locations within the cell structure. In this study the model metal-reducing bacterium Geobacter sulfurreducens was used to synthesise bio-Pd under different loadings of Pd(II) using different electron donors. This study combines ultramicrotomy and Scanning Transmission Electron Microscope (STEM) to establish the size distribution and location of bio-Pd within the cell structure. The cellular location of bio-Pd could not be identified from drop cast samples of whole cells, and the thickness of whole cells significantly affected imaging and particle size analysis. Ultra-thin sections (60 nm) provided a cross section of the cells, allowing imaging of the location of bio-Pd nanoparticles within the cell structure, and the thin, uniform samples improved imaging of nanoparticles.
Methods/Materials
Bio-Pd nanoparticles were prepared using G. sulfurreducens in bicarbonate buffer, supplied with either a low or high initial concentration of Pd(II), and either acetate, formate, or hydrogen as electron donor, or no electron donor (NED).
Samples were resin-embedded as previously [3], briefly, samples were washed and collected via centrifugation and cell pellets were fixed with glutaraldehyde, embedded in agarose, fixed again in glutaraldehyde, stained with reduced osmium, then dehydrated in graded ethanol and acetone. Samples were infiltrated with graded resin in acetone, followed by polymeristaion at 60oC for 3 days. Ultrathin sections (60nm) were cut with a diamond knife on an ultramicrotome. Thin sections were collected onto holey-carbon supported copper TEM grids. Drop cast samples were diluted in deionized water and 2 µL pipetted onto holey carbon supported copper TEM grids and allowed to air dry.
STEM imaging was performed in an FEI Talos F200A FEG TEM operated at 200 kV, or in an FEI Tecnai F30 FEG TEM operated at 300 kV, equipped high angle annular dark field (HAADF) detector using a convergence angle of 10.5 mrad and a detector angle of 40 – 200 mrad. Size analysis was performed on roughly 300 particles per sample in ImageJ, with the mean of two perpendicular line measurements taken per particle.
Results and Discussion
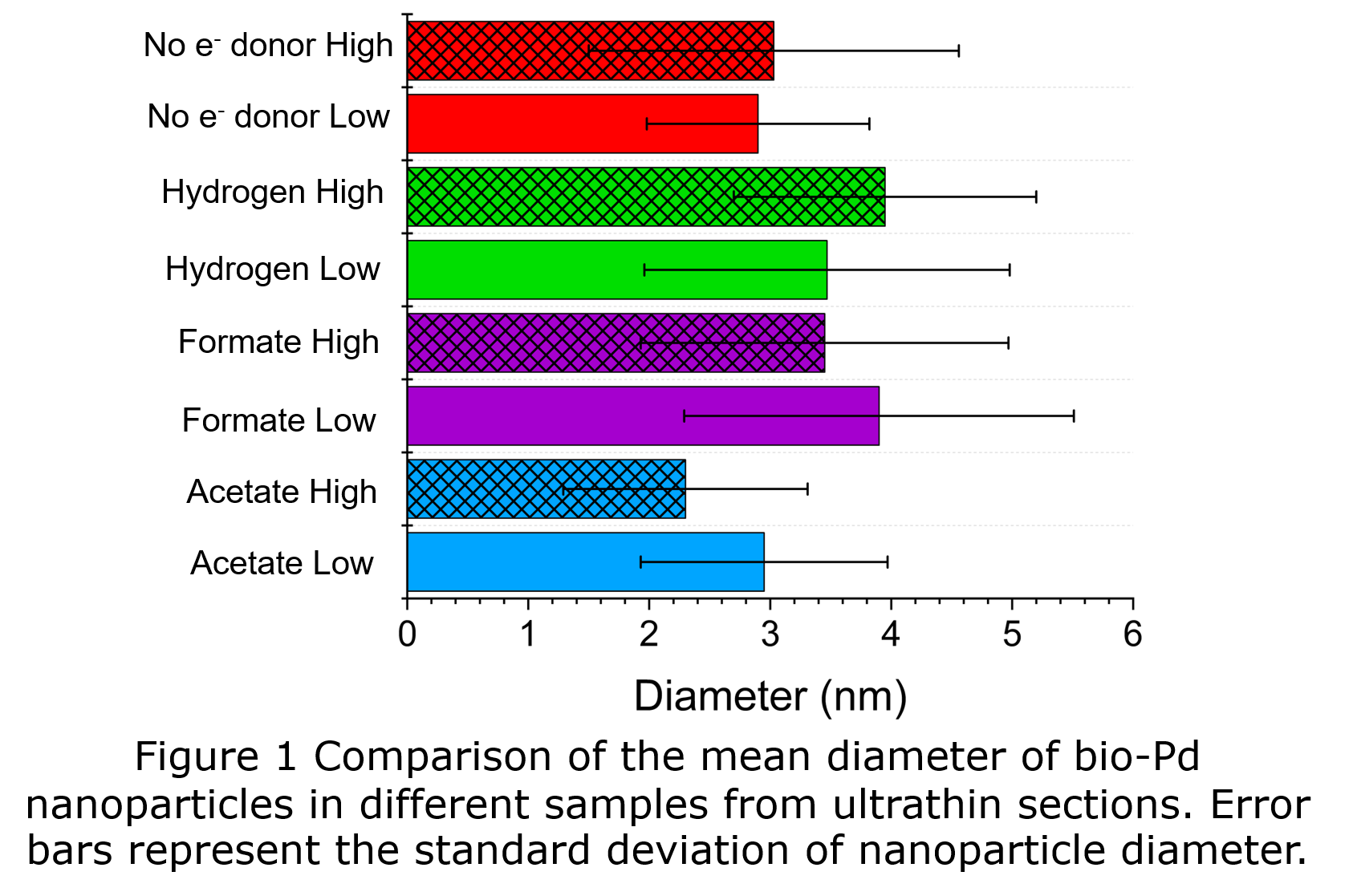
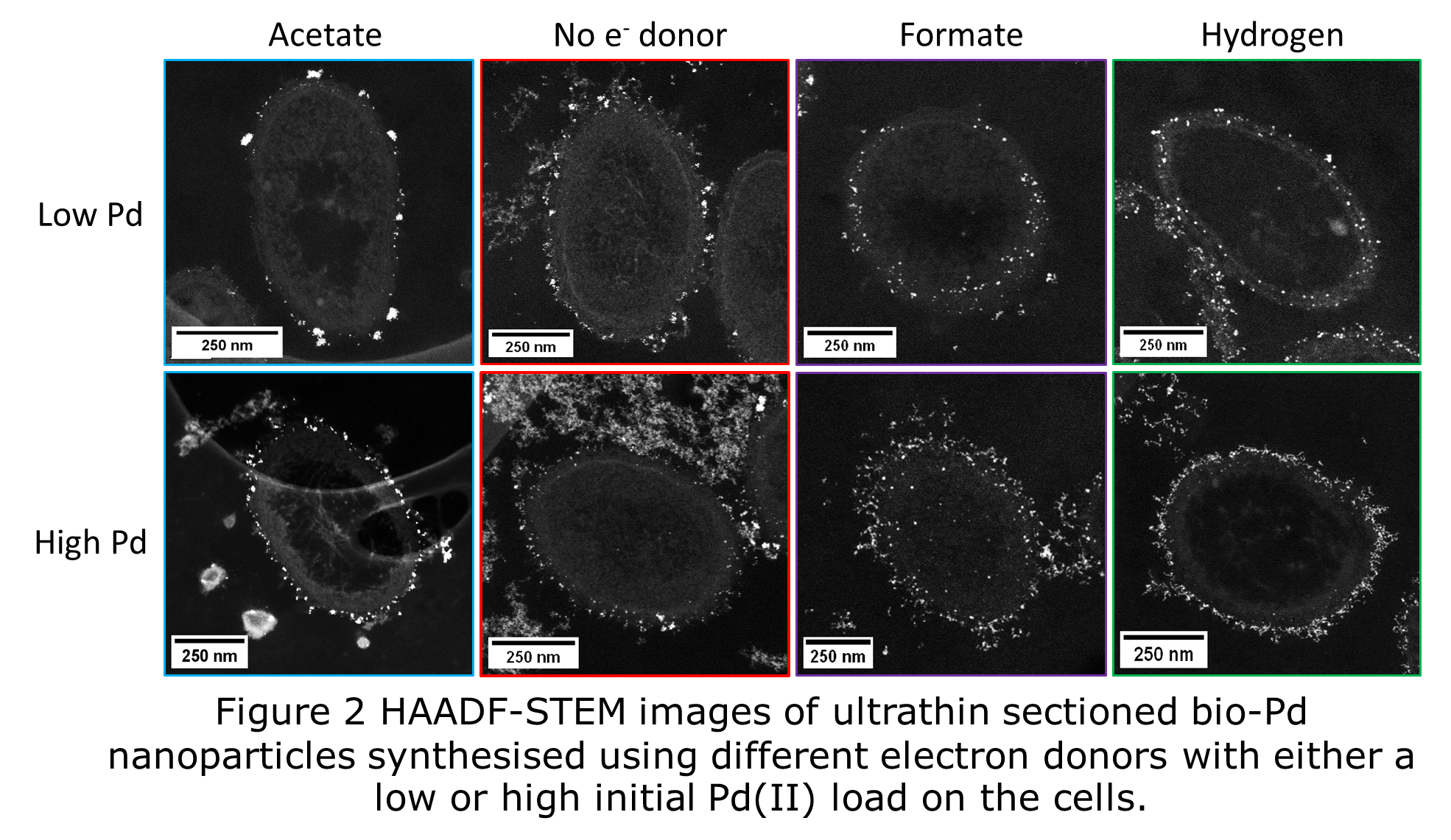
Size analysis of thin sections showed that all bio-Pd samples possessed a mean diameter of <4 nm (Fig 1). Cells of G. sulfurreducens supplied with acetate as electron donor or NED, produced bio-Pd with smaller size distributions than cells supplied with hydrogen or formate. Acetate and NED cells deposited bio-Pd at the outer membrane (Fig 2) as the extracellular electron transfer chain of G. sulfurreducens terminates with c-type cytochromes at the periphery of the cell. At high Pd load more extracellular particles were observed, which appeared to be bound by extracellular polymeric substances (EPS) (Fig 2) and were mostly <3 nm. When hydrogen or formate were supplied to cells at low Pd load the vast majority of bio-Pd was deposited between the inner and outer membranes (Fig 2), the location of the hydrogenase and formate dehydrogenase enzymes. However, at high Pd load bio-Pd was mostly deposited at the outer membrane. Hence both the choice of electron donor and the initial Pd(II) concentration affects the location of bio-Pd particles in the cell. Controlling the location of bio-Pd nanoparticles within the cell structure is useful as this will affect the availability of Pd(0) surface to reactants in catalysis. Extracellular and outer membrane particles are more available than particles between the inner and outer membrane, hence the catalytic properties will be different.
Conclusion
Ultramicrotomy and HAADF-STEM imaging revealed the location and size distributions of bio-Pd nanoparticles. The choice of electron donor and initial Pd(II) concentration supplied to cells of G. sulfurreducens enables control of the location of bio-Pd nanoparticles within the cell structure. All bio-Pd samples possessed mean diameter <4 nm, and cells supplied with acetate or NED contained the smallest particle size distributions.
- References
1. Lloyd, J.R., J.M. Byrne, and V.S. Coker, Biotechnological synthesis of functional nanomaterials. Current Opinion in Biotechnology. 2011. 22: p. 509-515.
2. Egan-Morriss, C., et al., Biotechnological synthesis of Pd-based nanoparticle catalysts. Nanoscale Advances, 2022. 4: p. 654-679. DOI: 10.1039/d1na00686j.
3. Kimber, R.L., et al., Biosynthesis and Characterization of Copper Nanoparticles Using Shewanella oneidensis: Application for Click Chemistry. Small. 2018. 1703145.