Characterization of metal oxide nanoparticles synthesized in encapsulins from Myxococcus xanthus
- Abstract number
- 251
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2023.251
- Corresponding Email
- [email protected]
- Session
- Poster Session Two
- Authors
- Mr Harry Benjamin McDowell (1), Dr Egbert Hoiczyk (1), Dr Thomas Walther (1)
- Affiliations
-
1. University of Sheffield
- Keywords
STEM, electron energy loss, energy-dispersive X-ray spectroscopy, high angle annular dark field, nanocompartments, nanoparticles, bacterial organelles, iron mineralization
- Abstract text
1. Summary
In this work, a combination of high angular annular dark field (HAADF), energy dispersive X-ray spectroscopy (EDS) and electron energy loss spectroscopy (EELS) are used to characterize the physiochemical properties of the iron containing mineral synthesized within the encapsulin from Myxococcus xanthus.
2. Introduction
The encapsulin a 32nm iron mega-storage complex from the bacteria Myxococcus xanthus. The complex is made up of two components: a shell (EncA) and three internalized cargo protein (EncB, EncC, EncD) whose functions remain unclear. Since the discovery of encapsulin, they have been the topic of intense research due to the ease with which the protein shell can be modified. However, little work has been undertaken to understand the iron containing material they mineralize. In this work, a combination of HAADF imaging, EDS and EELS will be used to characterize the iron containing mineral within the encapsulin shell. This work is important because it is one of the first works to try and characterize the material synthesized by these compartments.
3. Materials/Methods
Encapsulins were isolated from Myxococcus xanthus using the published protocol (McHugh et al., 2014). Further constructs were generated using an Escherichia coli overexpression system transformed with the petDUET-1 plasmid (Novagen) containing the shell protein and one of the three cargo proteins. The construct expressing stains were grown in an iron rich media to induce the mineralization of iron. The constructs were then purified using immobilized metal affinity chromatography. TEM and HAADF STEM images were taken using a JEOL F200 operated at 200KeV. EDS data was collected using dual silicon drift detectors. EELS data was collected using a Gatan Quantum GIF using DualEELs mode and a 0.25eV/ch energy dispersion. All spectral data collected was analysed using Gatan DigitalMicrograph 3.5.
4. Results
4.1 Iron concentration dependent nanoparticle growth
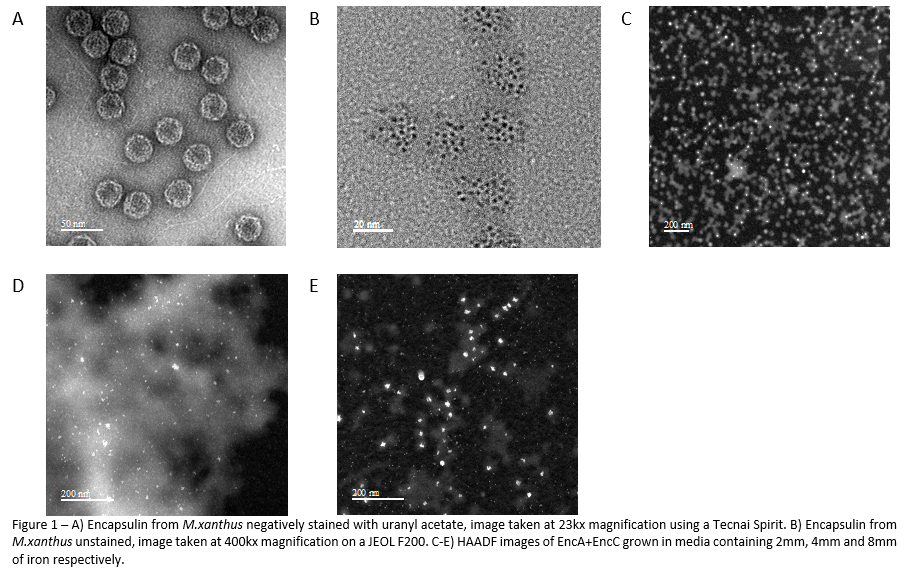
Encapsulin constructs grown in different iron concentrations mineralize iron differently. Figure 1B shows an unstained TEM image of the native encapsulin structure, here it can be seen that each shell contains several spherical particles 5nm in diameter. Constructs grown in 2mM and 4mM iron (Figure 1C-D) appear to synthesize particles with an amorphous shape. The construct grown in 8mM iron (Figure 1E) synthesis spherical particles ~5nm in diameter that are reminiscent of those found in the native structure, however some particles are much larger. The general distribution is a single particle per shell, but some shells appear to contain multiple particles.
4.2 The nanoparticles are comprised mainly of iron and oxygen.
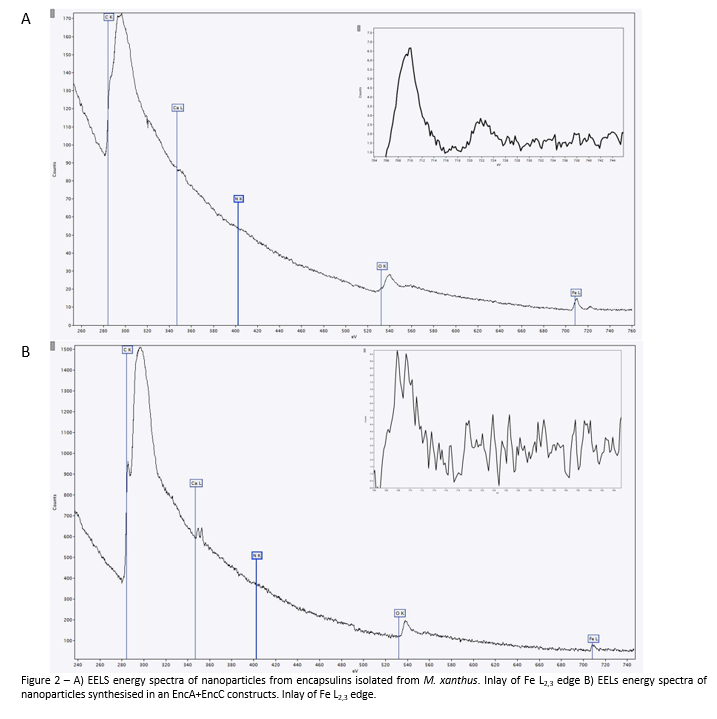
Analysis with EELS reveals peaks in the spectrum at 532eV and 708eV which represent iron and oxygen respectively. When the Fe L2 peak is examined, there are slight differences between the two samples. In the sample isolated from M. xanthus the L2 peak is shifted to 710eV suggesting the single valence state Fe3+ is present in the sample. Meanwhile the sample containing EncA+EncC shows a split L2 with peaks at 708eV and 710eV, this is indicative of a mixed valence material, composed of Fe2+ and Fe3+. Interestingly, there is another peak at 350eV which indicates calcium is also present in the material.
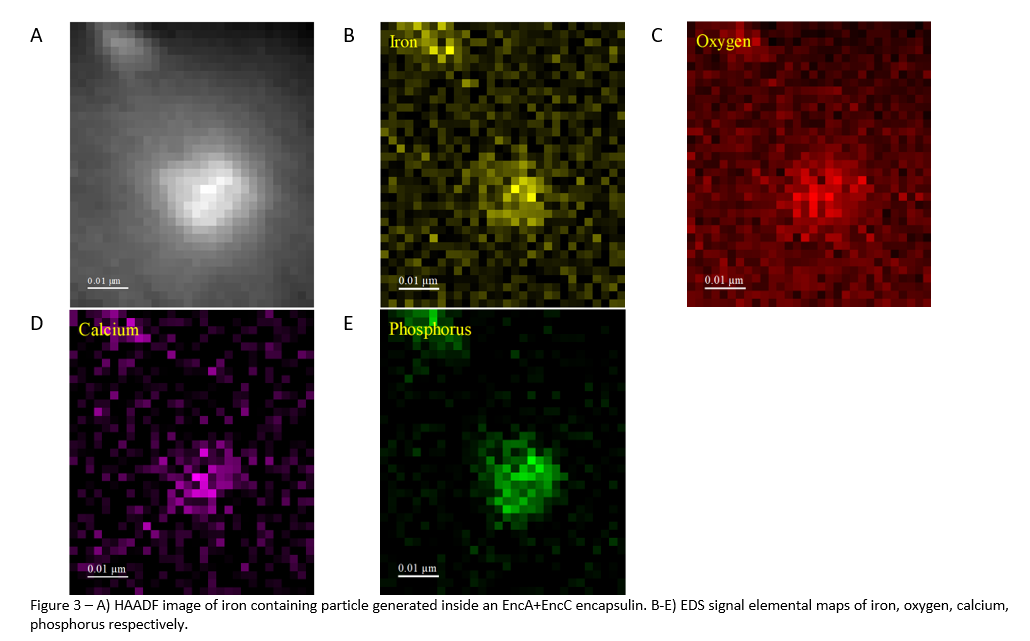
4.3 Elements are evenly distributed across the nanoparticle.
EDS chemical mapping shows that all elements identified in the EELS analysis are evenly distributed across the nanoparticle. The use of EDS imaging was also able to identify that phosphorous is a major component of the nanoparticle. This was not identified in the EELS analysis because its peak is outside the spectral range imaged.
5. Discussion
The results allow some conclusions to be drawn about the physical and chemical properties of the minerals synthesized inside the encapsulin nanocompartment. Firstly, we can identify the loading of the EncA+EncC compartments with iron is concentration dependent. As the concentration of iron is increased the particles change from amorphous to majority spherical. These 5nm spherical particles possess physical characteristics that are desirable and therefore are of great interest for future applications. Interestingly, a change in the iron concentration appear to result in nanoparticles with physical properties that differ from those isolated from the native bacteria. This may also be indicative of the internal iron concentration of the bacteria during encapsulin loading. The elemental analysis has revealed information about the chemical properties of these particles. Notably they are comprised of 4 elements: iron, oxygen, calcium and phosphorous. The presence of calcium is novel, as it is not commonly found complexed with iron by other iron mineralizing protein systems.
6. Conclusion
Overall, this work is significant as it provides an insight into the physical and chemical properties of a novel iron mineralizing system. Whilst also providing an insight into its potential usefulness as a platform for nanoparticle growth.
- References
McHugh, C.A. et al. (2014) “A virus capsid‐like nanocompartment that stores iron and protects bacteria from oxidative stress,” The EMBO Journal, 33(17), pp. 1896–1911. Available at: https://doi.org/10.15252/embj.201488566.