Characterising interaction between DNA and autophagy receptor NDP52 through the automated analysis of AFM images
- Abstract number
- 62
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2023.62
- Corresponding Email
- [email protected]
- Session
- Poster Session Two
- Authors
- Ms Mingxue Du (1), Mr Daniel E. Rollins (1), Dr Ália dos Santos (2), Dr Alice L. B. Pyne (1), Dr Chris Toseland (2)
- Affiliations
-
1. Department of Materials Science and Engineering, University of Sheffield
2. Department of Oncology and Metabolism, University of Sheffield
- Keywords
AFM, image analysis, DNA, NDP52
- Abstract text
DNA in the cell is tangled and twisted, adopts complex topologies, and is decorated with a myriad of DNA binding proteins which affect its structure and function1. Unlike the crystalline, linear DNA that led to the discovery of its double helical nature2, there is now a growing appreciation that the context of DNA in the wider genome is vital to its function3. To pursue a better understanding of the fundamental properties and functions of DNA, we must better understand how DNA behaves in its cellular environment, and how it responds to interactions with other oligonucleotides and proteins.
We have developed a pipeline to characterise changes in DNA conformation using a combination of atomic force microscopy (AFM) imaging and automated image analysis techniques. AFM is capable of achieving a double-helical resolution on DNA molecules within a physiological environment4. However, much of the data that this technique produces is not quantified due to a lack of automated tools. We have developed TopoStats5, software that can read raw AFM files, carry out initial, essential pre-processing, identify individual molecules of interest and analyse their structure.
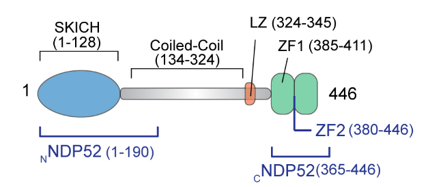
Here we study the interaction of DNA with the autophagy receptor protein NDP52, which was first discovered in the cell nucleus6 and has previously unknown roles in DNA shape regulation. It is predicted to exhibit a dumbbell shape (Figure 1)6. Though NDP52 was first discovered in the nucleus, previous studies were only able to identify functions related to cell adhesion and autophagy in the cytoplasm7,8. To date the role of NDP52 in the nucleus and the mechanism and function of interactions with DNA remain poorly understood. This lack of understanding can be attributed to the small size and flexibility of NDP52, which render it difficult for crystal structures to be obtained, and present challenges to the direct visualisation of its molecular structure.
Figure 1 Diagram of NDP52 illustrating its different components9
We set out to characterise the effect of NDP52 on DNA structure and investigate the potential nuclear roles of NDP52 using our pipeline of AFM combined with TopoStats. In order to determine protein structure and understand how this influences interactions, we characterise NDP52 and the complexes it forms by binding with DNA9.
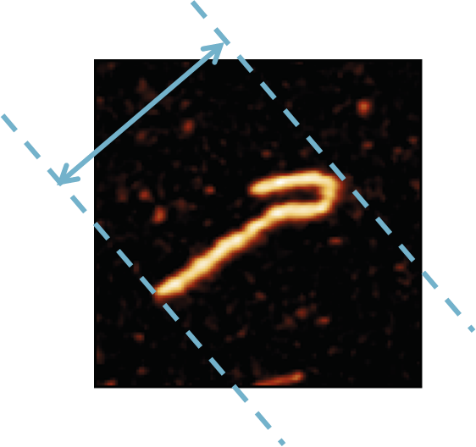
To process a large amount of data in a short time frame and avoid selection bias, we design and employ software for the automated analysis of AFM images5. This enables us to measure and compare the size distributions of full-length NDP52 and the C-terminal domain, and quantify the compactness of DNA molecules introduced through interaction with NDP52 by measuring the change in maximum bounding distance, as illustrated below (Figure 2).
Figure 2 Maximum bounding distance. This is the maximum distance between two parallel lines that touch the border of the molecule of interest.
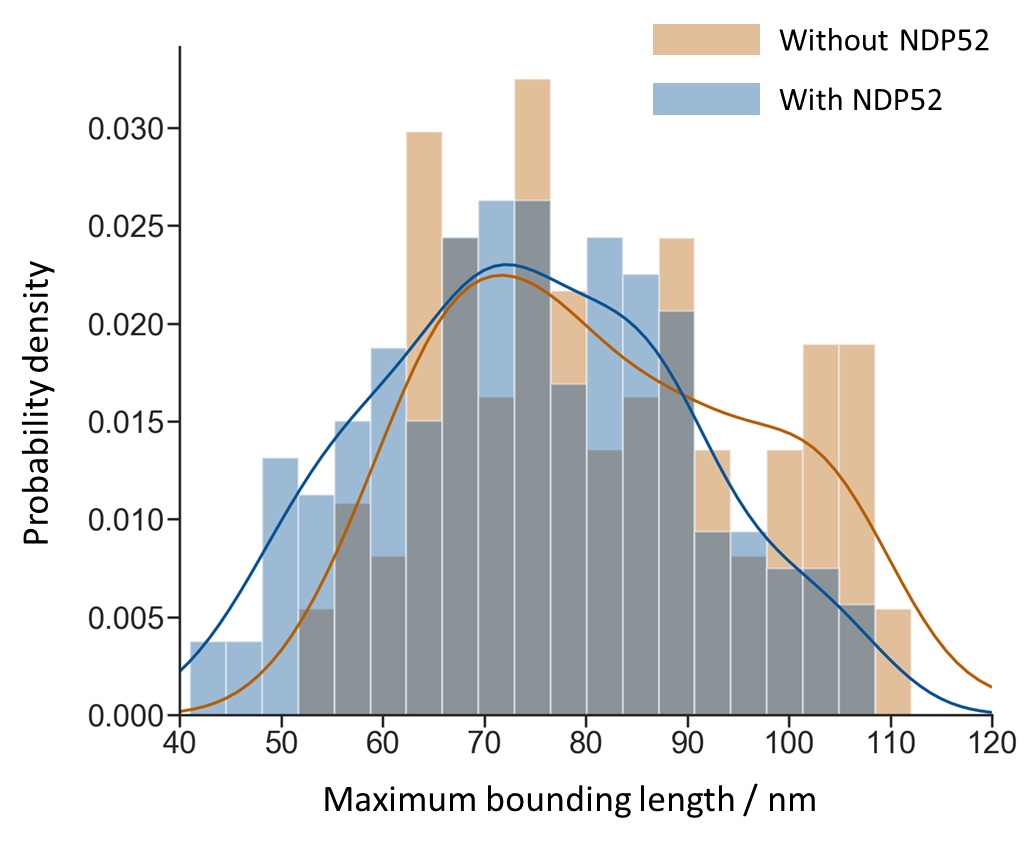
We observe the predicted dumbbell structure of NDP52, as well as bridging and looping in DNA induced by the binding of NDP52. We also show that the interaction of NDP52 causes a shift in the distribution of maximum bounding distance towards the lower end, which suggests a compaction of DNA shape (Figure 3). In particular, the first three bars of the histogram demonstrates that NDP52 can bend DNA beyond the possible extent of bending caused by other factors, such as the effects of immobilisation. This demonstrates that NDP52 alters DNA structure, and indicates that NDP52 does have a role in the nucleus.
Figure 3 The distributions of DNA maximum bounding distance with and without NDP52
Our results correlate with data obtained through other techniques such as size-exclusion chromatography with multi-angle light scattering and electrophoretic mobility shift assay, which together improve our understanding of the structure of NDP52 and confirm the presence of its oligomerisation. Combining these findings with the results of biochemical analysis, we propose that the nuclear functions of NDP52 are related to transcription initiation and DNA structure regulation.
- References
1. Bates, A. D. & Maxwell, A. DNA structure. in DNA Topology (Oxford University Press, 2005).
2. Franklin, R. E. & Gosling, R. G. Evidence for 2-Chain Helix in Crystalline Structure of Sodium Deoxyribonucleate. Nat. 1953 1724369 172, 156–157 (1953).
3. Fogg, J. M. et al. Bullied no more:when and how DNA shoves proteins around. Q. Rev. Biophys. 45, 257 (2012).
4. Pyne, A. L. B. et al. Base-pair resolution analysis of the effect of supercoiling on DNA flexibility and major groove recognition by triplex-forming oligonucleotides. Nat. Commun. 12, 1053 (2021).
5. Beton, J. G. et al. TopoStats – a program for automated tracing of biomolecules from AFM images. Methods (2021) doi:10.1016/j.ymeth.2021.01.008.
6. Korioth, F., Gieffers, C., Maul, G. G. & Frey, J. Molecular characterization of NDP52, a novel protein of the nuclear domain 10, which is redistributed upon virus infection and interferon treatment. J. Cell Biol. 130, 1–13 (1995).
7. Morriswood, B. et al. T6BP and NDP52 are myosin VI binding partners with potential roles in cytokine signalling and cell adhesion. J. Cell Sci. 120, 2574 (2007).
8. Mostowy, S. et al. p62 and NDP52 Proteins Target Intracytosolic Shigella and Listeria to Different Autophagy Pathways. J. Biol. Chem. 286, 26987 (2011).
9. Santos, Á. Dos et al. Autophagy receptor NDP52 alters DNA conformation to modulate RNA Polymerase II transcription. bioRxiv (2022) doi:10.1101/2022.02.01.478690.