Ac kelvin probe force microscopy enables nanoscale surface charge mapping in water
- Abstract number
- 166
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2023.166
- Corresponding Email
- [email protected]
- Session
- Poster Session Two
- Authors
- Thomas Hackl (1), Dr. Patrick Mesquida (1, 2), Mathias Poik (1), Univ.-Prof. Dr. Georg Schitter (1)
- Affiliations
-
1. Automation and Control Institute (ACIN) / TU Wien
2. Department of Physics / King's College London
- Keywords
AC-KPFM, surface charge, atomic force microscopy, microcontact printing, self-assembled monolayer, solid−liquid interface
- Abstract text
In this study, a novel atomic force microscopy (AFM) measurement mode (AC-KPFM) is developed and used to quantitatively map spatially distributed surface charges in aqueous environments at the nanoscale.
The measurement of nanoscale surface charges or the related surface potentials at the solid-liquid interface is of high importance in many areas, ranging from colloidal systems to biomolecular interactions. Classical methods, like dynamic light scattering or streaming-potential measurements, usually rely on a hydrodynamic model and do not provide spatial resolution. Kelvin-probe force microscopy (KPFM), an AFM based technique, on the other hand cannot be operated in aqueous environments. Here, the application of a DC-bias between the scanning cantilever probe and the sample induces various parasitic effects when operated in water (i.e. electromigration and electrolysis). With the recent development of AC-KPFM [1], this DC-Bias is replaced by a high-frequency AC-voltage, enabling its use for surface potential measurements in liquid environments [2].
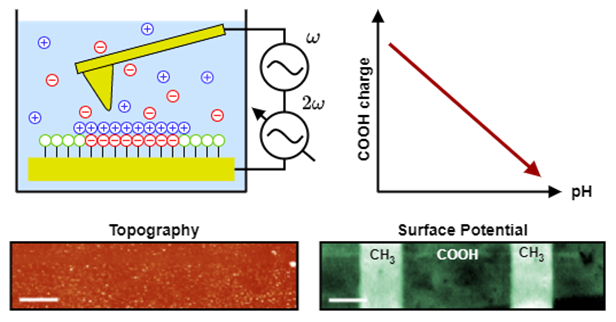
The schematic principle of AC-KPFM is shown in Figure 1, with its use of two high-frequency AC-voltages, whose controlled amplitude-modulation results in a measurement of the local surface potential underneath the cantilever tip. Structured thiol-monolayers, fabricated by microcontact-printing onto flat gold surfaces, are used as topography-free samples. This ensures pure electrostatic force contribution to the cantilever deflection, without unwanted crosstalk of topographical features. The alkanethiols are functionalized by amino- (NH2) and carboxy- (COOH) groups, whose protonation state (i.e. charge) is reversibly altered by the pH of the solution (COOH ↔ COO- / NH3+ ↔ NH2), as known from fundamental acid-base chemistry. Their potential distribution is measured by AC-KPFM in solution and related to the uncharged CH3 terminated thiols (see measured potential distribution of COOH/CH3 sample in Figure 1).
Figure 1: Working principle of AC-KPFM for surface potential measurements in aqueous solutions. The amplitude at 2𝜔 is controlled to nullify the cantilever deflection caused by the electrostatic interaction with the sample, resulting in a measurement of the local surface potential. The protonation state of the ionizable alkanethiol monolayers is altered by the solution pH and measured by AC-KPFM. (scale bar: 10µm)
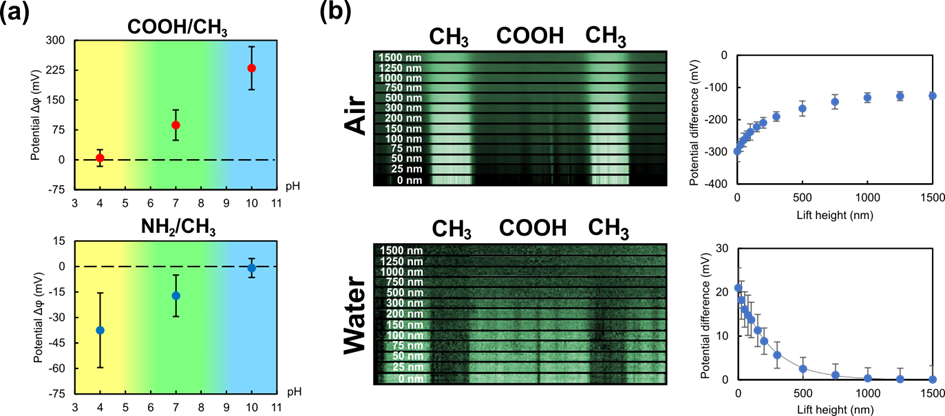
Figure 2(a) shows the measured potential difference from the ionized thiols (COOH or NH2) to the uncharged CH3 as a function of solution pH. A clear dependence of the measured potential on the pH is observable, validating the charge measurement capability of AC-KPFM in aqueous environments. Since the sample is immersed in aqueous media, an electric double layer (EDL) forms itself at the solid-liquid interface. It is found, that arrangement of counter-ions (i.e. the Stern-layer) near the surface impacts the measured potential and forms a deterministic tip-sample distance dependence when compared to measurements performed in air, as can be seen in Figure 2(b).
Figure 2: (a) Measured potential difference ∆𝜙 of the ionizable thiol (COOH / NH2) to the uncharged CH3 region as a function of pH. (b) Dependence of the measured potential difference on the tip-sample separation (lift-height) in air and deionized water.
The results show, that AC-KPFM enables the measurement of surface potentials in aqueous media and the investigation of the EDL with high spatial resolution, which is not possible with classical AFM-based or other methods. With the more recent development towards heterodyne detection [3] its spatial resolution is further increased, enabling quantitative determination of surface potentials of nanoscale samples, such as collagen fibrils. With that, many applications in biology or related fields are conceivable, such as the investigation of collagen glycation or histone acetylation.
- References
[1] D. Kohl, P. Mesquida, and G. Schitter, “Quantitative ac - kelvin probe force microscopy”, Microelectron. Eng., 176 (2017) pp. 28-32.
[2] T. Hackl, G. Schitter, and P. Mesquida, “Ac kelvin probe force miscopscopy enables charge mapping in water”, ACS Nano, vol. 16, no. 11 (2022) pp. 17982-17990.
[3] T. Hackl, M. Poik, and G. Schitter, “Heterodyne ac kelvin probe force microscopy for nanoscale surface potential imaging in liquids”, IEEE Trans. Instrum. Meas., (2023).