Optimizing each step of transmission electron microscopy from sample collection, processing, ultramicrotomy, and multiangle imaging improved the diagnosis of Primary Ciliary Dyskinesia.
- Abstract number
- 110
- Presentation Form
- Poster & Flash Talk
- DOI
- 10.22443/rms.mmc2023.110
- Corresponding Email
- [email protected]
- Session
- Poster Session Two
- Authors
- Ms. Shikha Chaudhary (1, 2), Dr. Kanaram Jat (1), Dr. Subhash Chandra Yadav (1)
- Affiliations
-
1. All India Institute of Medical Sciences
2. Amity Institute of Nanotechnology
- Keywords
Primary ciliary dyskinesia, Cilia, Transmission electron microscopy, Diagnosis
- Abstract text
Primary ciliary dyskinesia (PCD) is rare (heterogeneous, autosomal recessive) life-shortening respiratory tract disease caused by nonfunctional cilia in the airways with a prevalence of one per 20,000 births. The diagnosis of PCD by transmission electron microscopy (TEM) is finally revealed by detecting multiple ultrastructural defects associated with functionality/motility in cilia. Due to inappropriate (less amount) samples collection from pediatric patients, noncellular contamination (mucous), a smaller number of ciliary cells in samples, heterogeneity in ciliary ultrastructure in different cells of the same patients, and also, in the same cell, absence of simple ciliary cell enrichment, difficulties in primary cell culture, and requirement of at least 100-200 images of cilia with complete ultrastructure, the diagnosis of PCD through TEM is a herculean task. We have optimized the multistep procedure of TEM imaging from the sample collection (using a new kind of self-designed brush, cleaning the nasal cavity with saline before collection, and separate brushes for each nasal cavity) and quantification (within 30 min) of the number of cilia cells in each sample using carrier navigation and mosaic merge under a confocal microscope. Due to simple ciliary cell quantification methods, the samples containing 1:10000 ciliated cells were re-collected again on the same day. The noncellular contamination was removed using the saline and fixed in a newly standardized fixative (2.5% Glutaraldehyde, 2% Paraformaldehyde,0.05%Tannic acid,2mM MgSO4 in 0.2M Sodium Phosphate Buffer) to yield better results of cilia imaging. The ultramicrotomy was performed with minimal block trimming, and using single-hole grids improved the imaging of the higher number of cilia. The imaging at a different alpha angle improves the better visualization of cilia in higher numbers. Our standardized sample collection strategies (for more cilia cells with less mucus contamination), primary fixation (to preserve the cilia’s ultra-structures), ultra-sectioning (to increase the section area), and TEM imaging (by changing the alpha tilt) improve the reportability by each steps up to 30, 24, 14, and 30% respectively. Out of 300 analyzed clinical suspected samples (by video microscopy of a sample with abnormal cilia movement and clinical/molecular features), we were able to provide nearly 246 samples with at least 100 cilia (reportability), and finally, 80 patients were diagnosed with PCD using this methodology. This methodology may potentially develop a fast diagnostic of PCD using cryo-TEM and upon validating the clinical symptoms.
Establishing TEM-based diagnosis using this method may be helpful for the proper treatment of PCD with appropriate clinical guidelines.
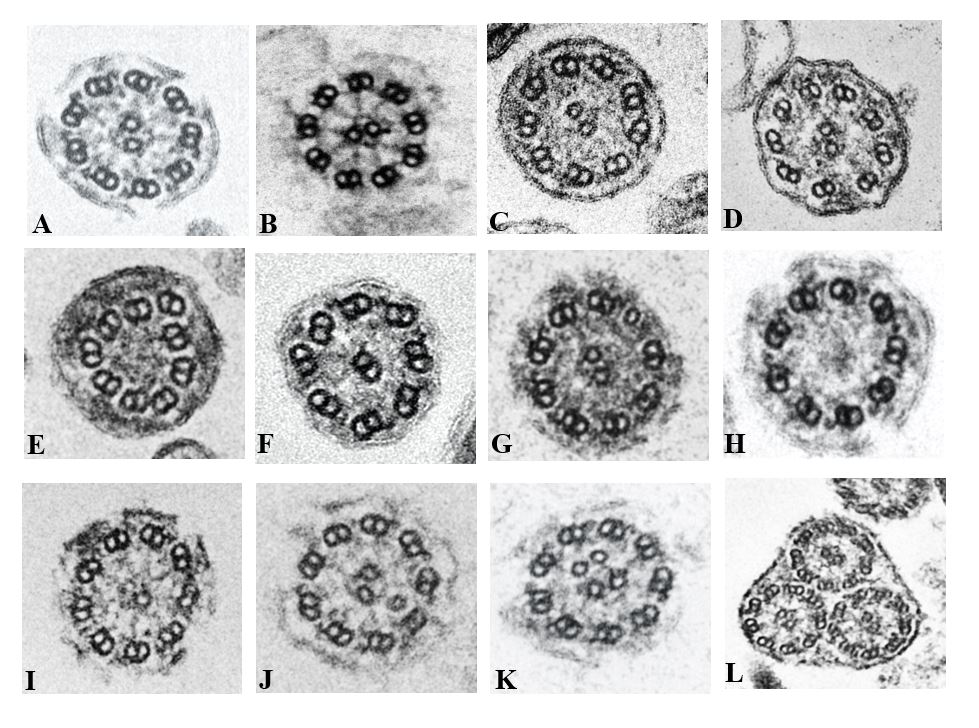
Figure 1: Ciliary defects reported in different patient samples at AIIMS New Delhi using optimized Transmission Electron Microscopy. (A) Normal cilia sections have a 9+2 microtubular structure with their associated inner and outer dynein arms surrounding the central microtubule pair. (B) Short Outer dynein arm (C) Missing inner dynein arms (D) Missing inner and outer dynein arms (E) missing inner and outer dynein arm as well as with no central microtubules (F) Dislocation of microtubules with missing inner dynein arm and no central microtubules (G) 8 doublet microtubules with 1 extra singlet microtubule (H) No central microtubule (I) Single central microtubule (J) Extra central microtubule. (K) Extra central microtubular pair (L) Compound cilia.
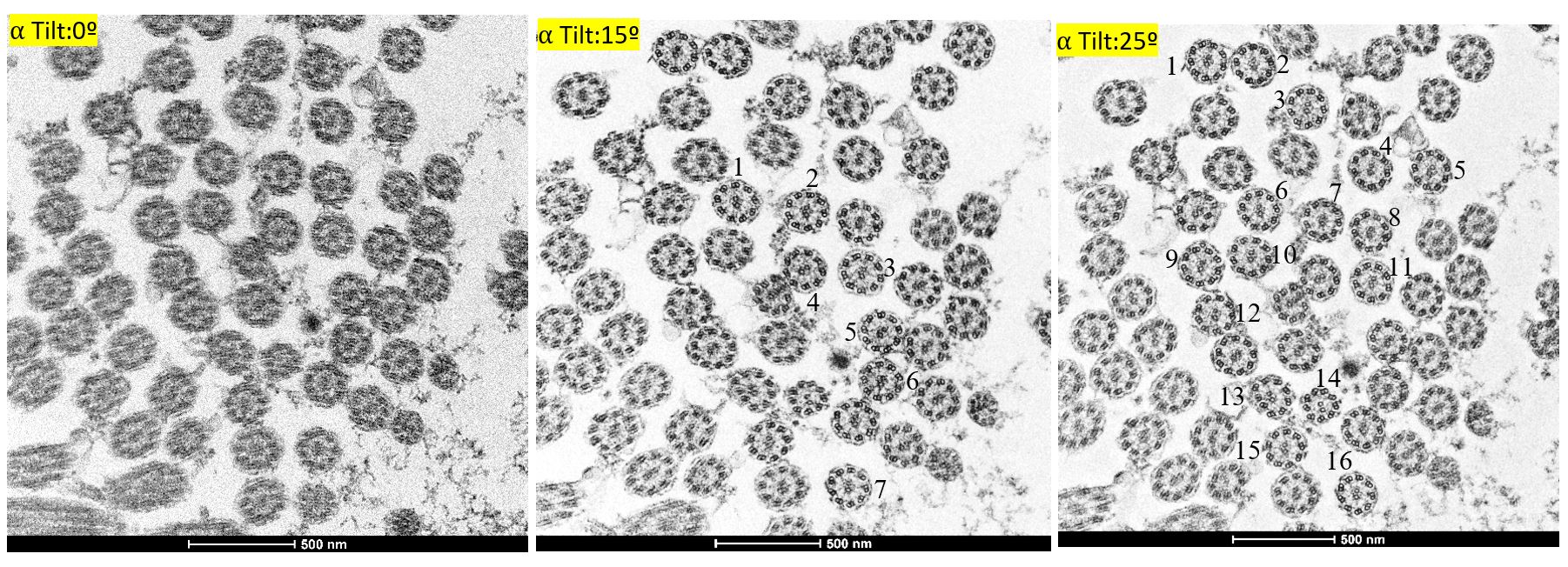
Figure 2: Impact of ⍺ tilting on the quality and quantity of the cilia imaging. (A) 0ᶛ, (B) 15ᶛ and (C) 25ᶛ revealed the increase in the quality and quantity of cilia from the same sample.
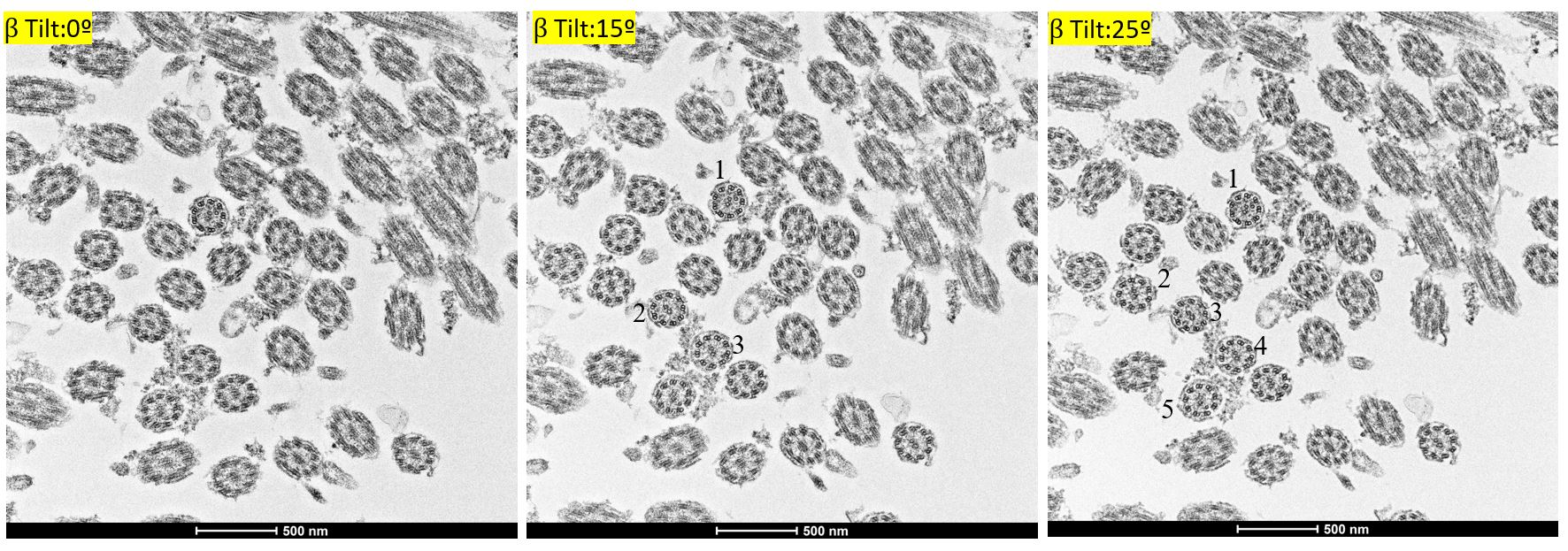
Figure 3: Impact of β tilting on the quality and quantity of the cilia imaging. (A) 0ᶛ (B) 15ᶛ and (C) 25ᶛ revealed the increase in the quality and quantity of cilia from the same sample.
- References