Collagen mineralization dynamics studied by in situ Raman spectroscopy and synchrotron X-ray scattering
- Abstract number
- 372
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2023.372
- Corresponding Email
- [email protected]
- Session
- Poster Session Three
- Authors
- Ms Emma Tong (2), Dr Julia Parker (1), Prof. Roland Kroeger (2)
- Affiliations
-
1. Diamond Light Source
2. University of York
- Keywords
Biomineralization
Bone Mineralization
In situ microscopy and spectroscopy
In situ Raman spectroscopy
In situ SAXS and WAXS
Electron Microscopy
Nano-XRF
- Abstract text
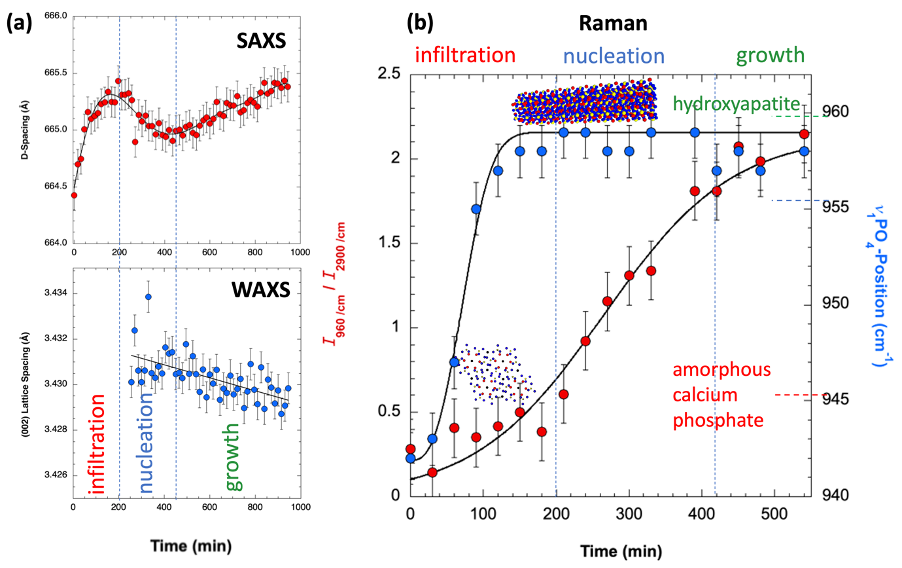
Collagen mineralization is a central process in bone formation. It is known that the control of the crystal growth, resulting in a fractal-like organization of the mineral phase within the collagen matrix, is essential for bone to obtain its unique mechanical properties [1]. However, the dynamics of the mineralization process remains a matter of intensive research. To shed light on this dynamics we applied the polymer-induced liquid precursor (PILP) process [2] using osteopontin as an active protein polymer to mimic this process in vitro using rat-tail derived collagen fibers. Mineralization was carried out in situ in a custom-made heatable flow-cell allowing for Raman microspectroscopy as well as small-angle and wide-angle X-ray scattering characterization (SAXS, WAXS). Raman spectroscopy enables a molecular level analysis of the mineralizing collagen, SAXS provides information on the particle evolution and impact of mineral growth on the collagen D-banding (a periodic pattern caused by repeat sequences of overlap and gap regions in the staggered collagen molecule organization) and WAXS permits the study of the time dependent evolution of crystallinity monitoring the {002} lattice planes in the hydroxyapatite mineral phase. The resulting mineralized collagen fibers were subsequently analysed using nano-X-ray fluorescence (nano-XRF) microscopy to map the distribution of Ca and P within the fibers and transmission electron microscopy (TEM) for microstructural investigations. The in vitro mineralization experiments show significant similarities to the mineralization patterns observed in bone with the Raman and SAXS/WAXS studies being in strong agreement (see Fig. 1).
Fig. 1: (a) In situ SAXS/WAXS studies of OPN mediated collagen mineralization as a function of time. (b) In situ Raman studies using the same flow cell and growth conditions as in (a).
The mineralization pattern observed by nano XRF is shown in Fig. 2 and reveals a tessellation motif similar to the one reported for human and murine bone with the crystal morphologies observed by TEM indicating a similar crystal organization pattern compared to that found in bone [3].
Fig. 2: (a) Overlay of Ca and P maps of in vitro mineralized collagen as measured by nano-XRF showing tessellation (Ca: red, P: green). (b) TEM image revealing calcium phosphate crystals within the mineralized collagen.
This further confirms a strong similarity of the physico-chemical processes controlling collagen mineralization in our in vitro system compared to that found for bone (see Fig. 2). Monitoring the collagen D-banding and mineralization progress as a function of time, we reveal the occurrence of at least one intermediate mineral phase within the first two hours of mineralization and a change of the D-banding indicating the infiltration, nucleation and growth phases of the calcium phosphate formation process within the collagen matrix.
- References
[1] N. Reznikov, M. Bilton, L. Lari, M. Stevens, and R. Kröger, Fractal-like hierarchical organization of bone begins at the nanoscale, Science 360, science.aao2189 (2018).
[2] M.J. Olszta, D.J. Odom, E.P. Douglas, L.B. Gower, A new paradigm for biomineral formation: mineralization via an amorphous liquid-phase precursor, Connective Tissue Research 44 (1), 326 (2003).
[3] D. Buss, N. Reznikov, M. McKee, Crossfibrillar mineral tessellation in normal and Hyp mouse bone as revealed by 3D FIB-SEM microscopy, J. Struct. Biol. 212 107603 (2020).