The MoTraScope: A Dual View motion tracking Microscope
- Abstract number
- 563
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2023.563
- Corresponding Email
- [email protected]
- Session
- Poster Session Three
- Authors
- Ms Amy Hassett (1), Ms Niamh Burke (1), Dr Mark Pickering (1)
- Affiliations
-
1. University College Dublin
- Keywords
motion tracking, behaviour tracking, dual view microscope
- Abstract text
Summary
Here we describe the Motion Tracking Microscope (MoTraScope), a dual-view imaging platform capable of detecting and tracking the position of a target (e.g., small, freely behaving marine invertebrates) within a sample chamber. A low magnification camera system captures the wider centimetre-scale context of a target, while a high magnification microscope mounted on a motorised XY stage images micron-scale details of the target. We developed this device specifically for tracking the movement of small animals within an experimental arena while maintaining a microscopic view of the animal during behaviour.
Introduction
Historically, approaches to studying the behaviour of small animals have relied upon either placing the animal in a highly constrained environment and capturing the fine details of its behaviours or capturing a relatively low dimensional description of the position and motion of an animal within an unconstrained environmental arena (Pérez-Escudero, 2014). To provide a comprehensive description of the behaviour of a small animal, information about the detail and the context of the animal’s behaviour is required.
Achieving this using a single optical device is difficult because optical microscopy typically involves a trade-off between resolving power and field of view, where increased spatial resolution comes at the cost of contextual information. However, by employing two optical devices - one with a large field of view that captures the entire experimental arena, and one high magnification device which captures the fine details of the animal’s behaviour, we can overcome this limitation.
Description of the MoTraScope
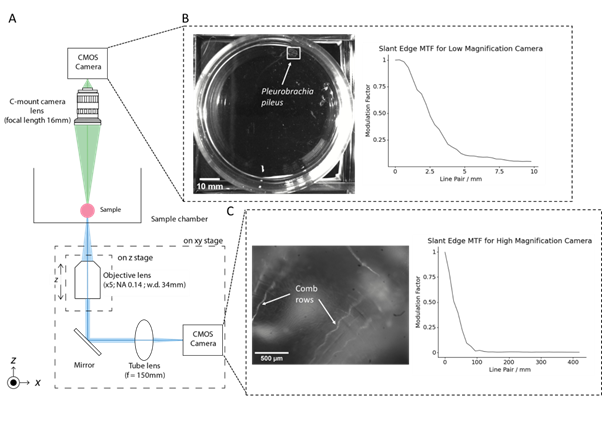
We have developed an imaging platform called the Motion Tracking Microscope (MoTraScope), which includes these two camera systems (Figure 1A). The low magnification view consists of a CMOS camera (FLIR Flea3, VITA1300 sensor) and a 16mm C-mount camera lens mounted 335mm above the sample plane. This gives a field of view of 131mm x 104mm and allows for the identification and tracking of a target within this field of view.
The high-magnification microscope is attached to a motorised stage, which can then follow and capture micron-scale details of the sample. This assembly consists of a 5x objective lens (Mitutoyo Plan Apo Infinity Corrected Long WD Objective, NA = 0.14) with a working distance of 34 mm, mounted to a focussing stage with 1.27cm of travel. Light from the objective travels to a 45° turning mirror which directs the light through an f=150mm achromatic doublet to the camera (Allied Vision Alvium 1800 U-291m, Sony IMX421 sensor). The field of view of the high magnification microscope is 2.33mm x 1.76mm. The motorised XY stage has a travel range of 116mm by 99mm and is capable of movements up to 13.12mm/sec.
To track a target, we used OpenCV, a library of computer functions used for real-time computer vision (Bradski, 2000). OpenCV object tracking algorithms used the feed from the low-magnification camera to detect and track a target (Figure 1B). The position of the target was then converted to G-code and sent to the motorised XY stage. This repositioned the high-magnification microscope such that the target remained in the field of view (Figure 1C).
Figure 1: The MoTraScope is a dual-view camera system capable of detecting and tracking the movement of a target within a sample chamber and capturing a high-resolution image of the target. (A) Schematic of the MoTraScope, with light paths of the low and high magnification optical systems shown in green and blue respectively. The low magnification camera has a wide field of view and is used to detect the location of a target within the sample chamber, in this case, the marine invertebrate Pleurobrachia pileus (B, left panel). A custom Python script, utilizing OpenCV tracks the location of the animal (marked by a white box) in the field of view of the low-magnification camera (C, left panel). With the high magnification view, greater detail of the features of the animals can be seen including the body wall of the animal, and the comb rows (the locomotory structure of Pleurobrachia). (B, C right panels) The performance of each optical device in the MoTraScope was assessed using slant edge MTF, by taking an image of a printed black square for the low magnification camera and by taking an image of a USAF 1951 resolution testing grid (B, left pane). The MTF profiles of both camera systems were computed using the SE_MTF_2xNyquist.jar ImageJ plugin.
Performance of the MoTraScope
The MoTraScope was stable at rest, drifting less than 30μm in both the X and Y directions over 10 minutes. The difference between the requested and observed movement of the XY stage was 3.23±0.43% and 2.06±0.51% (mean±SD) over repeated movements in X and Y respectively.
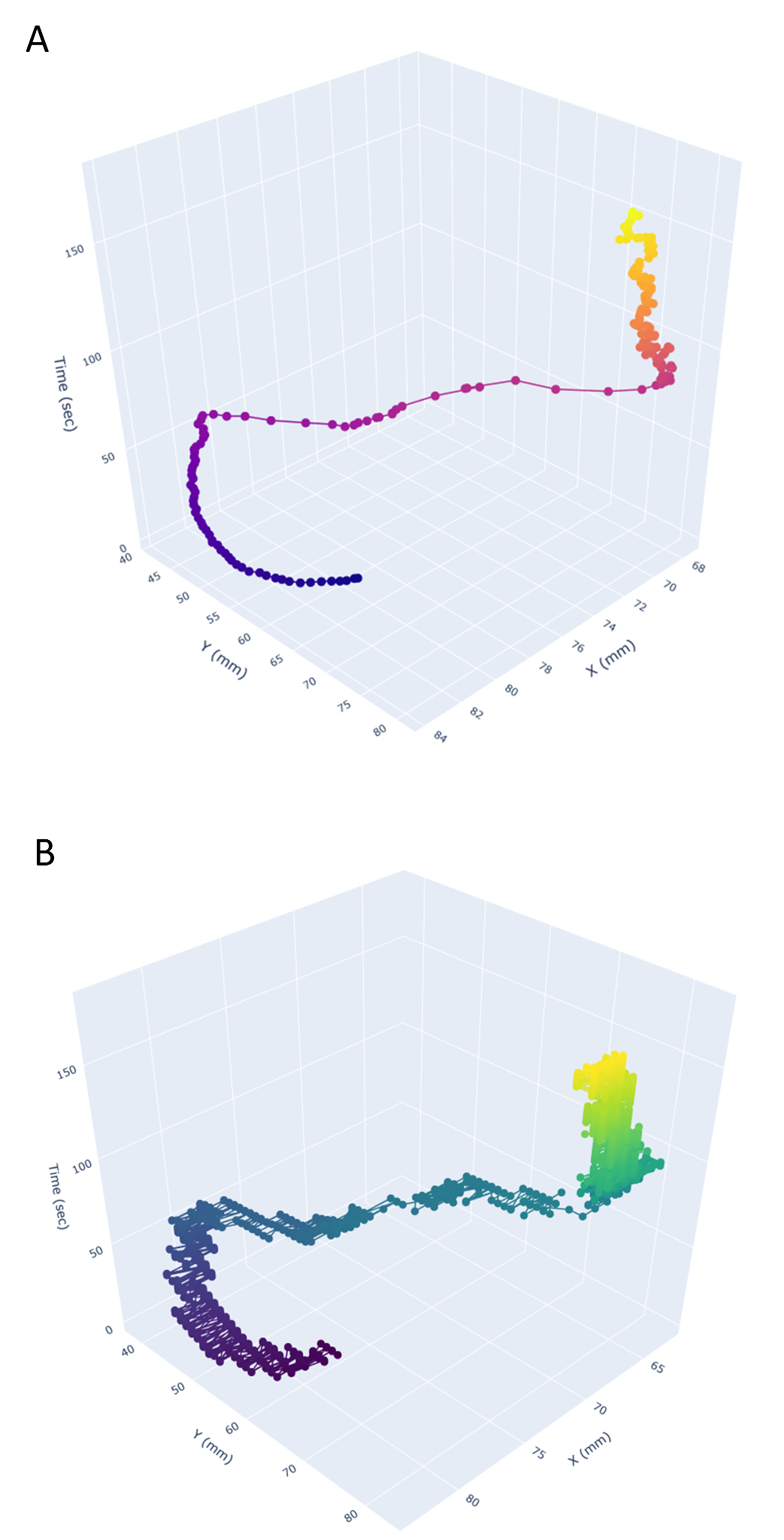
When we placed an individual Pleurobrachia (a small marine invertebrate) into the sample chamber, the movements of the MoTraScope matched the position of the animal over the course of a three-minute recording (Figure 2). Therefore this device is able to track the movement of an animal within an experimental arena and capture a microscopic view of the animal.
Figure 2: The MoTraScope is capable of detecting and tracking the location of a target of interest. (A). The position of an individual Pleurobrachia within the sample chamber and (B) the position of the high magnification microscope over the course of a three-minute recording. The position of the high-magnification microscope closely followed that of the animal.
Conclusion
The MoTraScope is an optical device capable of reliable simultaneous high and low-magnification imaging and motion tracking, thus enabling the tracking of an animal within an experimental arena while capturing a microscopic view of the animal during behaviour.
- References
Bradski, G., 2000. The OpenCV Library. Dr. Dobb’s Journal of Software Tools.
Kennedy, A. (2022). The what, how, and why of naturalistic behavior. Current Opinion in Neurobiology, 74, p.102549. doi:https://doi.org/10.1016/j.conb.2022.102549.