Activation of 2D polymerisation with atomic quantum clusters on insulating and metal surfaces
- Abstract number
- 84
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.84
- Corresponding Email
- [email protected]
- Session
- Atomic and Molecular Resolution Phenomena via AFM, STM and Scanning Probes
- Authors
- Alessio Quadrelli (1), Leonardo Forcieri (1), Qingqing Wu (1), Songjun Hou (1), Barry Mangham (3), Neil Champness (2), David Buceta (4), Manuel Arturo Lopez-Quintela (4), Colin Lambert (1), Samuel Paul Jarvis (1)
- Affiliations
-
1. Lancaster University
2. University of Birmingham
3. University of Nottingham
4. University of Santiago de Compostela
- Keywords
molecules, 2D materials, 2D polymers, on-surface polymerisation, high-resolution AFM, simulations,DFT, x-ray photoelectron spectroscopy, atomic quantum clusters, catalysis, insulating surfaces, metal surfaces
- Abstract text
On-surface polymerisation is routinely used to produce a wide variety of covalently stabilised 1D and 2D molecular structures at surfaces1. Despite rapid progress in this field, the fabrication of these polymers on non-metal surfaces remains comparatively slow, particularly using thermal methods. Without a catalyst, precursor molecules sooner desorb from insulating surfaces before they can polymerise, greatly limiting our ability to prepare surface polymers on non-metal substrates where their properties could be better exploited.
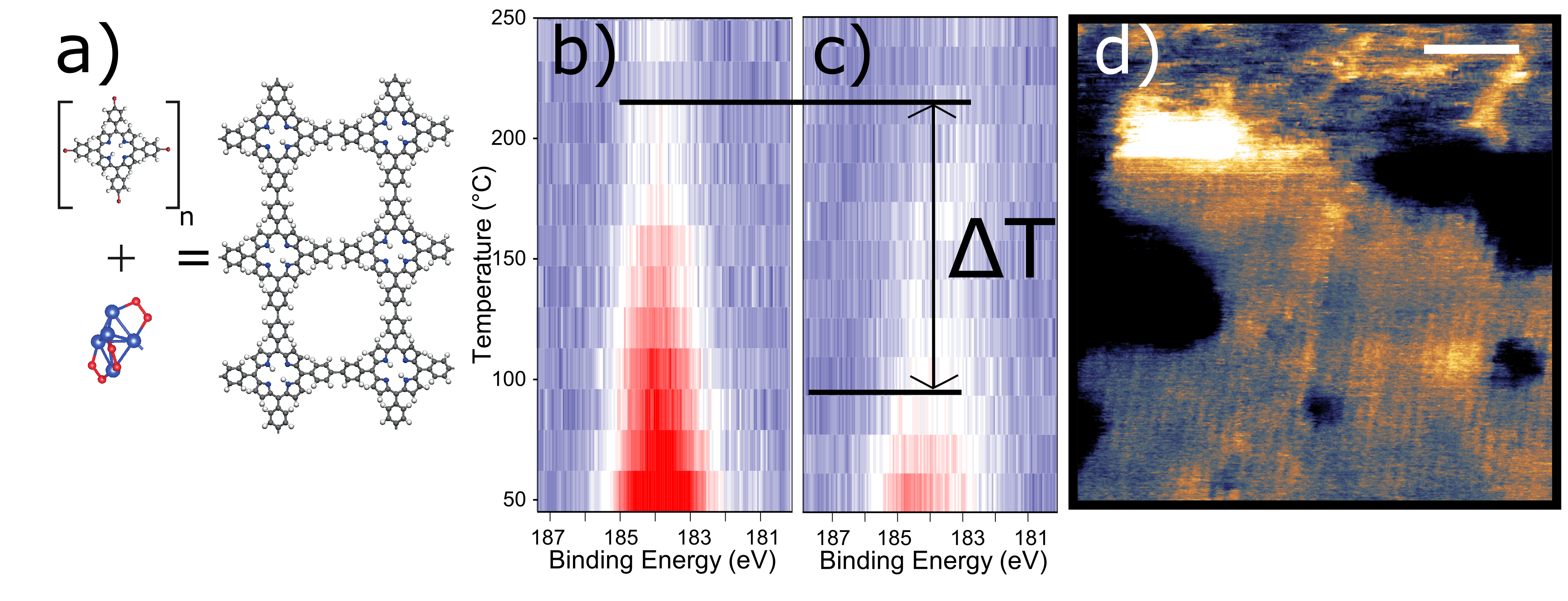
Here we report on-surface 2D-polymerisation on insulating and metal substrates using catalytically active copper atomic quantum clusters (AQCs). These unusual AQCs consist of just five copper atoms stabilised by an oxide layer2 (Cu5[O2]n), which we characterise using a combination of AFM, XPS, and normal incidence X-ray standing wave (NIXSW). Following this, we report the polymerisation of tetra (4-bromophenyl) porphyrin (Br4TPP) (Figure 1a) on mica and Au(111) substrates. Temperature-programmed X-ray photoelectron spectroscopy (TP-XPS) measurements show that Cu5[O2]n clusters substantially reduce the activation temperature for polymerisation, resulting in a polymer layer that survives well beyond the desorption temperature of the single monomers (Figure 1b,c). Polymer formation is supported by high-resolution AFM in ambient conditions (Figure 1d) and by extensive density functional theory (DFT) and climbing image nudged elastic band (CI-NEB) calculations which reveal a strong dependence between the catalytic activity of Cu5[O2]n AQCs and their stabilizing oxygen layer.
These results provide a new route for activating on-surface polymerisation on insulating surfaces that could be extended to other surfaces such as SiO2 and TiO2. Furthermore, we establish ambient condition AFM as a tool to investigate the reaction products in systems inaccessible by Scanning Tunnelling Microscopy.
Figure 1. (a) Schematic of Br4TPP polymerisation catalysed by Cu5[O2]n. TP-XPS plot of the Br 3p region for Br4TPP on mica (b) without and (c) with Cu5[O2]n atomic clusters, respectively. The addition of Cu5[O2]n reduces the dehalogenation temperature by ΔT=120±30 °C. (d) High-resolution AFM image of Br4TPP polymer on mica prepared with Cu5[O2]n clusters acquired in ambient (scalebar equals 10 nm).
- References
1. Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
2. Huseyinova, S. et al. Synthesis of Highly Stable Surfactant-free Cu5 Clusters in Water. J. Phys. Chem. C 120, 15902–15908 (2016).