Comparison of Structure and Li intercalation Properties in Natural and Artificial Graphite Materials as the Anodes in Li-ion Batteries.
- Abstract number
- 122
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.122
- Corresponding Email
- [email protected]
- Session
- EMAG - Energy Materials
- Authors
- Mr Ioannis Siachos (2, 3), Dr Zachary Ruff (1, 3), Prof. Dame Clare Grey (1, 3), Prof. B. Layla Mehdi (2, 3)
- Affiliations
-
1. University of Cambridge
2. University of Liverpool
3. The Faraday Institution
- Keywords
Lithium ion battery, (S)TEM, degradation, SEI formation, carbon anodes, graphite, Ni-rich cathodes, energy storage.
- Abstract text
Lithium-ion batteries (LIBs) play a crucial role in our life due to their unparalleled combination of energy density, rechargeability and power density [1, 2]. Graphite has been the key member as a lithium-ion host structure for the anode part of LIBs for the last few decades, benefiting from its high energy and power density, low cost, extended cycle life, high abundance and high theoretical capacity (372 mA h g-1) [3]. Extensive research studies have been made to find alternatives to increase the overall capacity of the negative electrode such as the mix of graphite and silicon [4]. However, it is crucial to understand the difference between natural and artificial graphite, and address knowledge gap in the difference of their electrochemical performances as the lithium intercalation host, as well as structural changes in respective solid electrolyte interfaces (SEI) during cycling [5].
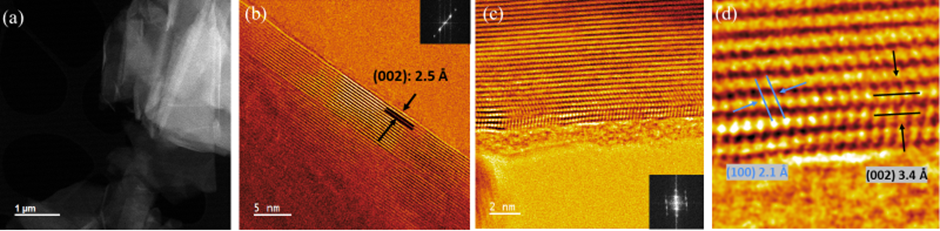
In this work, we investigate the structural characteristics and Li intercalation properties of natural and artificial graphite. We report ex situ X-ray diffraction (XRD) analysis, (scanning) transmission electron microscopy ((S)TEM) and electron energy loss spectroscopy (EELS) characterization; before and after cycling of both the negative electrodes. The natural graphite has a mixture of rhombohedral symmetry (3R) and 2H (2 layers per hexagonal (H) unit cell) with ABCABC stacking sequence which is less thermodynamically stable than the ABABAB stacking sequence of the artificial graphite with just 2 layers per hexagonal (H) unit cell [6]. These different structural properties are also confirmed through the XRD results where the presence of 3R is only visible in the natural material and absent in the artificial one. The ex situ high resolution (S)TEM images (Figure 1) show that the graphite (002) lattice planes remain unaffected after charge and confirms the presence of amorphous carbon which appears near to the high crystalline region of artificial graphite.
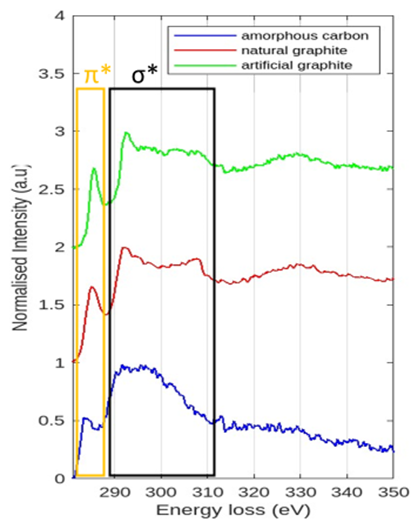
Furthermore, to compare the performance of these two graphite materials as the anode part we need to understand the intercalation mechanism of Li+ ions into the graphite anodes and the solid electrolyte interface (SEI) layer formation. We performed EELS to observe the π* and σ* edges of the pristine, cycled natural and artificial graphite anodes. The elemental mapping of carbon and other possible elements of the SEI layer, such as oxygen, fluorine, and phosphorus, can be observed from the energy-dispersive X-ray spectroscopy (EDS) analysis. The SEI layer is forming along the basal plane of graphite, which is consistent with the ex situ (S)TEM observation. Future work will focus on the study of the SEI composition by cryo FIB-SEM and (S)TEM.
Figure 1: (a) Ex situ TEM image of pristine naturally occurring graphite. (b) TEM image shows the (002) graphitic layers of the natural material. (c) DF STEM image of pristine artificially occurring graphite showing the presence of amorphous carbon near to the high crystalline region of artificial graphite. (d) DF STEM of the individual C atoms from the edge planes of the naturally occurring graphite.
Figure 2: EELS spectrum shows the pristine natural (red) and pristine artificial (green) to appear with more intense π* peak (yellow square) and much sharper σ* peak (black square) than the amorphous carbon region (blue) due to the presence of edge and basal planes. Also, it confirms the absence of any form coating on both of the graphite anodes.
- References
[1] H. Zhang et al, Energy Storage Materials, 36 (2021), p. 147-170 10.1016/j.ensm.2020.12.027
[2] J. Asenbauer et al, The Royal Society Chemistry 4 (2020), p. 5387-5416 10.1039/D0SE00175A
[3] M. Li et al, Advanced Materials 30 (2018), p. 1800561 10.1002/adma.201800561
[4] S. Chae et al, Angewandte Chemie International Edition 59 (2020), p. 110-135 10.1002/anie.201902085
[5] S. J. An et al, Carbon, 105 (2016) p. 52-76 10.1016/j.carbon.2016.04.008
[6] A. Eldesoky et al, Journal of The Electrochemical Society, 168 (2021), p. 110543 10.1149/1945-7111/ac39fc
[7] Supported by the EPSRC UK Faraday Institution under the Degradation (FIRG01) project