Complexities of liquid cell transmission electron microscopy and how we might start to overcome them
- Abstract number
- 380
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.380
- Corresponding Email
- m.a.ilett@leeds.ac.uk
- Session
- EMAG - In-situ EM Techniques & Analysis
- Authors
- Dr Martha Ilett (1), Dr Zabeada Aslam (1), Mr Nisarg Makwana (1), Professor Rik Drummond-Brydson (1)
- Affiliations
-
1. University of Leeds
- Keywords
Transmission electron microscopy
Liquid cell TEM
Fluid dynamic modelling
- Abstract text
This project aims to combine experimental and modelling data to better understand the complexities and limitations of liquid cell transmission electron microscopy (LCTEM) and begin to find ways to overcome these.
The use of liquid cell microscopy within materials science has become increasingly popular over the last decade. It allows analysis of dynamic processes observed in the liquid state without the need for any drying or freezing processes [1]. However, it is now understood that there are many complexities to LCTEM particularly in terms of the chemistry of the liquid and how it changes under the high energy electron beam [2]. Furthermore, the very confined volume afforded in most designs can cause microfluidic problems with regards to appropriate flow and mixing of solutions in dual flow systems [3]. Here we aim to better understand flow, mixing and electron beam interactions within LCTEM to allow improvements in experimental design and ultimately observe a truer representation of dynamic reactions.
For the experimental work in this project, we used a Hummingbird Scientific liquid cell holder alongside an FEI titan Themis TEM operating at 300 kV equipped with a Gatan one-view CCD. A dispersion of 1 mg/mL ZnO nanoparticles (<300 nm primary particle size) was prepared either in water or phosphate buffer solution (PBS). For imaging, scanning TEM (STEM) was used with an electron probe current <100 pA and dwell time <20 µs. In parallel to experimental work, we used COMSOL to model the liquid fluidic system in the LC holder in order to (i) identify optimal flow rates for full mixing within the chip window and (ii) observe the flow velocities through the system and across the viewing window.
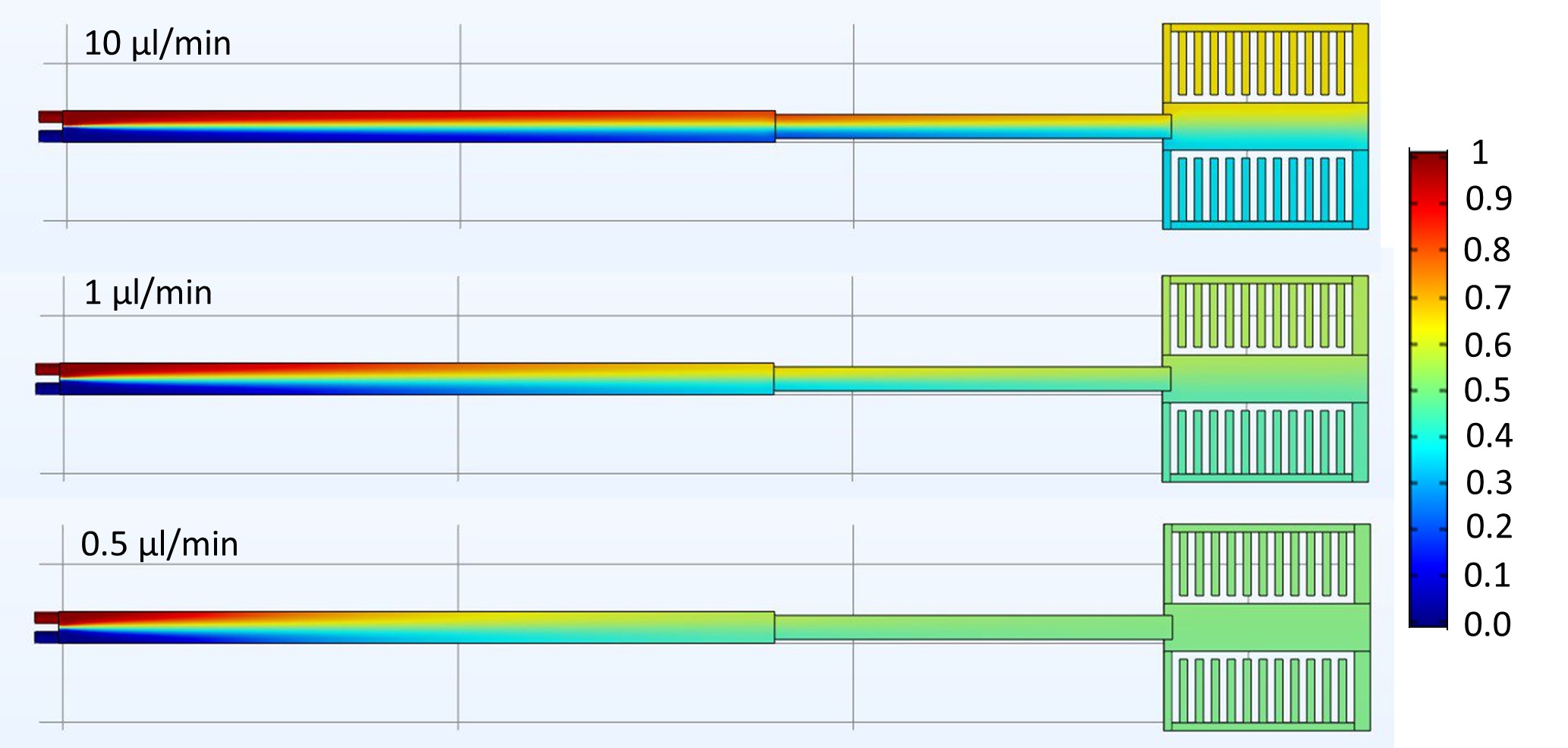
We used ZnO nanoparticles dispersed in water as a marker of pH change within the LC under the electron beam. ZnO dissolves at pH <7 and we observed full dissolution of ZnO after exposure to an electron fluence of just 10 e-/Å2. This suggests the onset of pH decrease within the LC is very rapid. In comparison ZnO was dispersed in PBS (a neutral buffer) and loaded in the LC. After imaging under the same beam conditions ZnO in PBS required exposure to >1000 e-/Å2 to fully dissolve. This illustrates the buffering effect possible inside the liquid cell in order to negate some of the radiolytic effects of beam illumination. In addition, we observed that by decreasing the dwell time from 20 µs to 1 µs, dissolution (in water) can be delayed. To further understand the complexities of LCTEM we have used COMSOL to model the fluid flow and mixing within the confined liquid environment. Significantly we have shown that full mixing is only obtained at very slow flow rates of <1 µl/min (Fig. 1). Furthermore, through microfluidic modelling we have observed that flow is favoured in the space around the window and indeed flow over the window channel might not the primary flow pattern.
Ultimately the observations and recommendations here will provide the field of LCTEM with suggestions regarding appropriate experimental design in order to obtain the truest representation of dynamic processes being investigated within the controlled liquid environment.
Figure 1: COMSOL modelling of diffusional mixing in liquid cell at varying flow rates. Flow rates below 1 µl/min are optimal for complete mixing of two solutions. The colour bar refers to mixing coefficient where 0.5 (green) is two solutions fully mixed.
- References
[1] Aoon Rizvi et al., Observation of Liquid–Liquid-Phase Separation and Vesicle Spreading during Supported Bilayer Formation via Liquid-Phase Transmission Electron Microscopy, Nano Lett. 2021, 21, 24, 10325–10332
[2] Schneider et al., Electron–Water Interactions and Implications for Liquid Cell Electron Microscopy, J. Phys. Chem. C 2014, 118, 38, 22373–22382
[3] Guomin Zhu et al., Addressing some of the technical challenges associated with liquid phase S/TEM studies of particle nucleation, growth and assembly, Micron, 2019, 118, 35-42