Determining the structure and chemistry of alloyed nanoparticles for hydrogen fuel cells using advanced electron microscopy and Density Functional Theory
- Abstract number
- 535
- Presentation Form
- Contributed Talk
- Corresponding Email
- [email protected]
- Session
- New and Emerging Concepts in Microscopy
- Authors
- Alessandro Zanre (1), Aakash Varambhia (2), Dogan Ozkaya (2), Rebecca Nicholls (1), Peter Nellist (1)
- Affiliations
-
1. Department of Materials, University of Oxford
2. Johnson Matthey
- Keywords
STEM, atomic resolution, DFT, strain, catalysis, Hydrogen fuel cells
- Abstract text
The use of hydrogen is increasingly seen as a key component to meeting our goals on net zero carbon energy sources. Hydrogen fuel cells are used to convert hydrogen to electricity, but are limited by the sluggish oxygen reduction reaction at the fuel cell cathode. Catalysts based on platinum are used to increase this reaction rate, but Pt is an expensive metal limiting the economic viability of hydrogen-based energy. Alloying Pt with cheaper base metals reduces the mass of Pt required, and surprisingly can enhance activity beyond that of Pt. The origins of the enhanced activity are not fully understood and may be associated with compositional clustering of species, the chemical effects of mixing metals or the effect of lattice strain if the composition is inhomogeneous. The challenge is that measuring either strain or composition within a nanoparticle is right at the limits of current experimental capabilities, especially as we need methods that can examine many particles to understand the ensemble properties.
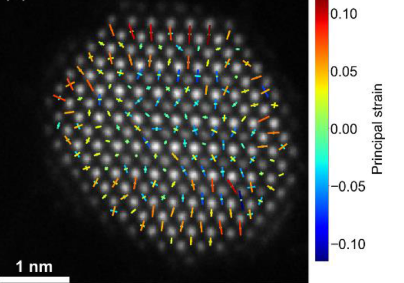
Previous work has used a combination of HAADF-STEM, EDX, and EELS to measure both composition and strain of Pt-Co catalyst nanoparticles at atomic resolution.1 At the surface of these nanoparticles a combination of tensile radial and compressive circumferential strains are observed, leading to a strain state with a high amount of shear. Normal strains have been shown to influence the d band energy of catalyst surfaces, which in turn influences their catalytic activity in the oxygen reduction reaction;2 however, similar investigations on the effect of shear strain on catalytic activity have not yet been performed. In this work we further explore the link between strain and catalytic performance using Density Functional Theory, and validate these findings by comparison with experimental data.
Figure 1: A map of principal strains measured on atomic columns of a PtCo3 catalyst nanoparticle using HAADF-STEM. Reproduced from [1].
- References
[1] : Luo, X. et al. (2022) ‘High-precision atomic-scale strain mapping of nanoparticles from STEM images’, Ultramicroscopy, 239, p. 113561. Available at: https://doi.org/10.1016/j.ultramic.2022.113561.
[2] : Sarwar, M. et al. (2020) ‘Exploring fuel cell cathode materials using ab initio high throughput calculations and validation using carbon supported Pt alloy catalysts’, Physical Chemistry Chemical Physics, 22(10), pp. 5902–5914. Available at: https://doi.org/10.1039/D0CP00301H.