Development of a photo-manipulation system combining holographic optical tweezers, patterned illumination and photoablation with real-time confocal microscopy
- Abstract number
- 78
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.78
- Corresponding Email
- cz374@cam.ac.uk
- Session
- Imaging Biomechanics
- Authors
- Chengxi Zhu (1), Helena Crellin (2), Neza Vadnjal (2), Ruby Peters (2), Martin O. Lenz (1), Ewa K. Paluch (2), Clare E. Buckley (2), Kevin O’Holleran (1)
- Affiliations
-
1. Cambridge Advanced Imaging Centre, University of Cambridge
2. Department of Physiology, Development & Neuroscience, University of Cambridge
- Keywords
Photo-manipulation, optical design, holography, optical tweezers, light patterning, photoablation live-cell imaging, confocal microscopy, force measurement, photoactivation.
- Abstract text
In recent years, research interest has focused on the development of photo-manipulation systems to switch molecular behaviours, control biospecimen status, and measure their properties quantitatively, which requires a broad range of techniques involving optogenetics, photobleaching, photoactivation, photoablation and optical trapping. To our knowledge, a singular system that encompasses all of these photo-manipulation techniques has yet to be realised, owing to the technical challenges of designing such a complex optical set-up.
Here, we have developed a versatile optical system utilising a confocal unit, holographic optical tweezers, a patterned illuminator and an ablation system to overcome these technical challenges. The confocal unit combines a state-of-the-art spinning disk system equipped with a high-power, multi-line laser bank for excitation. The holographic optical tweezers utilise a NIR laser and a high-resolution spatial light modulator to undertake optical trapping. The patterned illuminator is based on a digital micromirror device for simultaneously photobleaching and photoactivation. The photoablation system is based on a pulsed femtosecond Ti:Sapphire laser to ablate the biospecimen within a customised region of interest instantly.
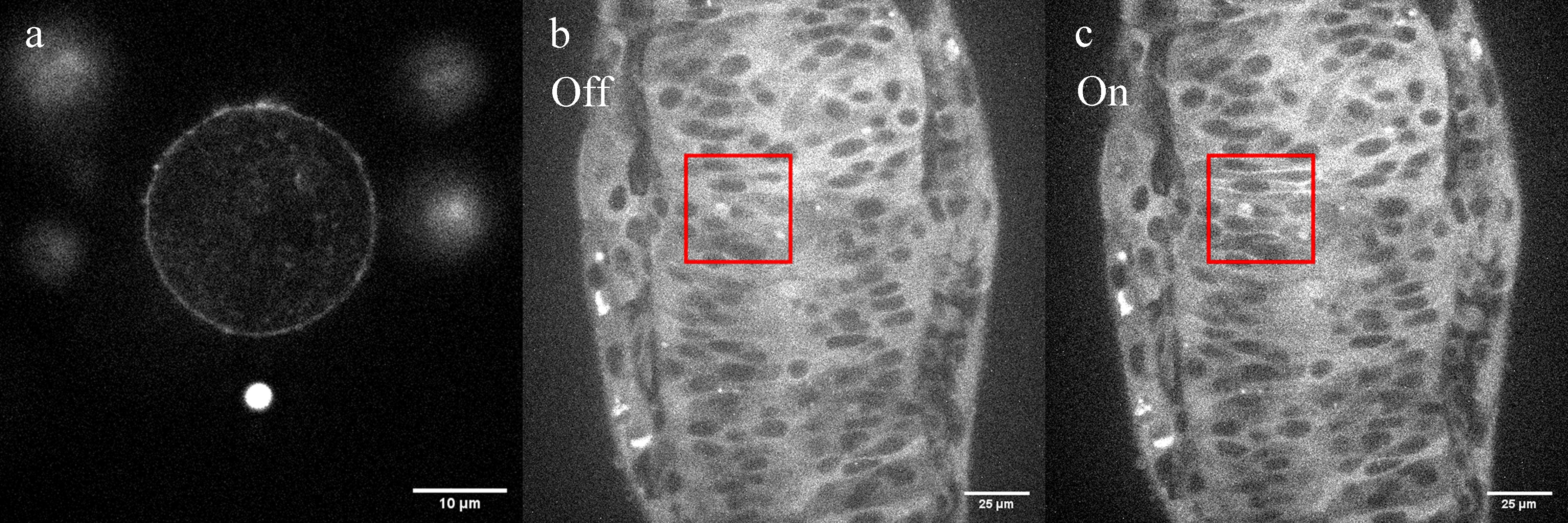
The broad applicability and versatility of our photo-manipulation system is presented in two biological examples: Imaging of live HeLa cells, quantifying the cell membrane tension and studying cell shape governed by F-actin dynamics and organisation (Figure 1a), and the in vivo imaging of cell behaviour and protein localisation in zebrafish embryos combined with highly spatially and temporally specific induction of reversible optogenetic protein-protein interactions (Figure 1b-c). This unique combination of fluorescence imaging, holographic optical tweezers, patterned illumination and photoablation opens up a whole new spectrum of applications, such as studying the cellular response to the local ablation and investigating the sufficiency of signalling or scaffolding proteins by manipulating their subcellular localisation. The presented photo-manipulation system is a powerful tool to address key questions in cell biology, from the single cell level to the entire embryo level.
Figure 1. Applications of the presented photo-manipulation system in optical trapping and photoactivation modes. (a) Live-cell confocal image of F-actin (SiR-Actin) in HeLa cells. For membrane tension measurements, a tether was generated between the cell (excited by 640 nm) and the fluorescent bead (excited by 405 nm). (b) and (c), Confocal images (GFP) of an optogenetic tool injected in a zebrafish embryo. Within the region of interest (depicted as a red square), the status of cells were switched from deactivated (b) to activated (c), leading to an increase in the brightness of the cell membranes.