Direct Observation of Zinc Dendrite Growth in Zinc Air Battery by Operando (S)TEM
- Abstract number
- 275
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.275
- Corresponding Email
- [email protected]
- Session
- EMAG - Functional Materials
- Authors
- Mr Xiaodong Liu (1), Professor Nigel Browning (1, 2), Professor Layla Mehdi (1, 2)
- Affiliations
-
1. Department of Mechanical, Materials & Aerospace Engineering, University of Liverpool
2. Albert Crewe Centre, University of Liverpool
- Keywords
Operando (S)TEM, zinc air battery, dendrite growth, nucleation
- Abstract text
Advanced rechargeable batteries are in great demand, which are expected to break through the limitations of the current energy conversion and storage (ECS) technologies. In recent years, the rechargeable metal air batteries (MAB) have attracted increasing attention due to the low cost and high energy density [1]. Compared to the typical metal air batteries (Li-air, Al- air), rechargeable zinc air batteries (ZAB) [2-3] are quite promising for future applications owing to the abundant zinc reserves as well as the outstanding large-scale productivity [4]. However, one of the key obstacles for the practical application of zinc air battery is the formation of zinc metal dendrite during the cycling process [5]. The fast growth of zinc dendrite often leads to serious short-circuiting issues and thereby reduce the cycling life of rechargeable zinc air batteries. Multiple approaches were adopted to restrain the zinc dendrite growth through either optimizing the electrolyte or modulating the electrode. However, due to the limitations of the characterization technologies, most studies only focused on illustrating the cycling properties, whilst the underlying enhancing mechanisms were still awaiting exploration. Therefore, it is of great importance to gain in-depth understanding and control of mechanism and intermediate stages of the zinc dendrite growth to increase battery safety.
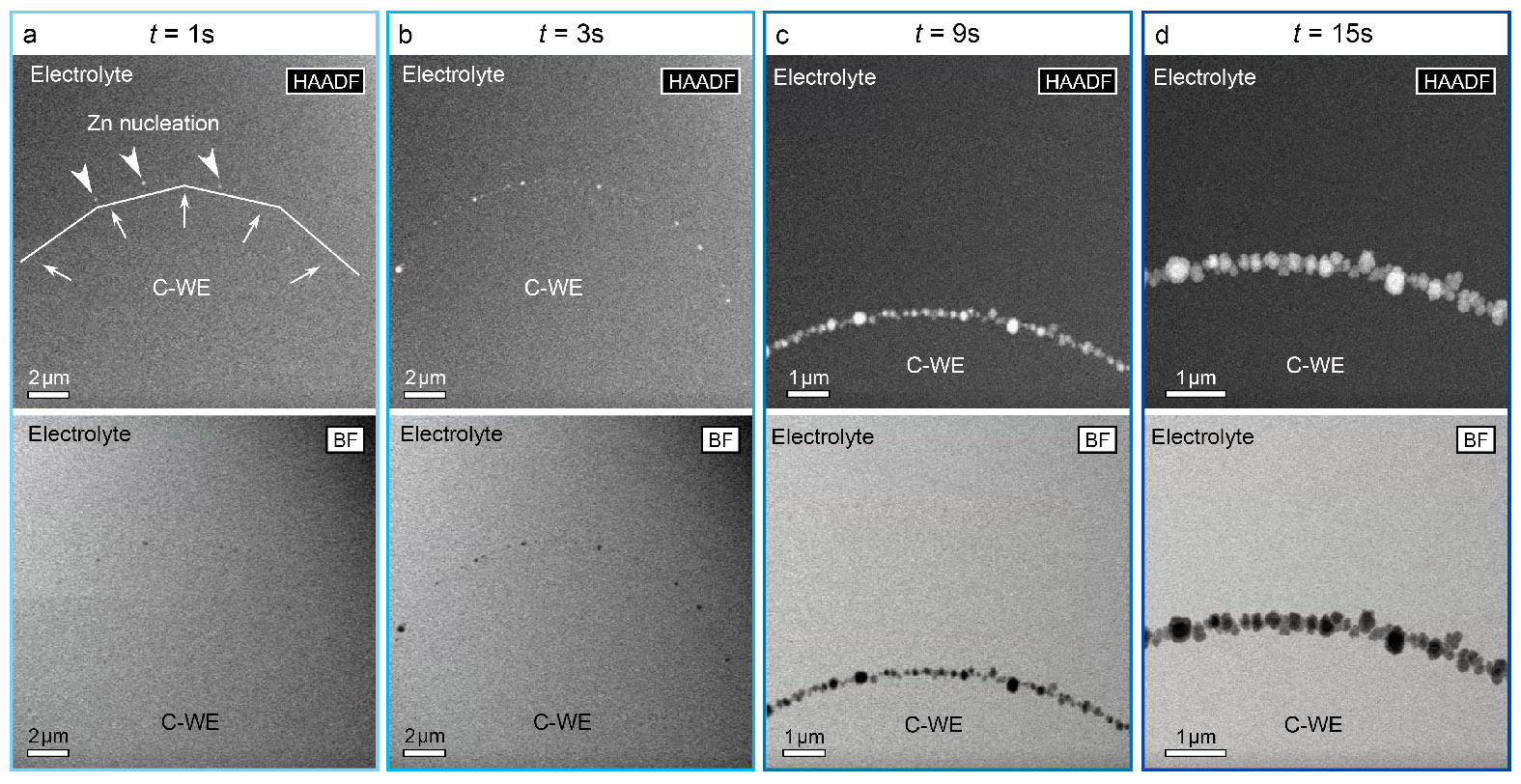
In this work, we employed an operando electrochemical liquid (S)TEM cell [6] to reveal the dendrite growth mechanism in nano zinc air batteries. Figure 1 shows the direct observation of dynamic dendrite growth processes at liquid/solid interface and enables us to understand the characteristics including the initial nucleation routes, growth speeds, preferences and orientations. In combination with the chemical characterization methods such as EDX and EELS, the chemical species and reactions at the interface between the electrode and the aqueous electrolyte were also analysed. This investigation provides new understandings of zinc dendrite evolution process and offers insight into the degradation mechanism of zinc air batteries, which could guide the future development of new rechargeable zinc batteries.
Figure 1. Sequential HAADF (top) and BF (bottom) STEM images of the Zn dendrite nucleation and growth during the electrodeposition process at the carbon working electrode (C-WE) at 10 mV/s scan rate. (a) t = 1s; (b) t = 3s; (c) t = 9s; (d) t = 15s.
- References
[1] M. Rahman et al. Journal of The Electrochemical Society, 160(2013), p. 1759–1771.
[2] J. Fu et al. Advanced Materials, 31(2019), p. 1805230.
[3] P. Gu et al. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 5(2017), p. 7651–7666.
[4] O. Caballero-Calero et al. Advanced Sustainable Systems, 5(2021), p. 210009.
[5] N. Shang et al. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 1(2022), p. 16369–16389.
[6] L. Mehdi et al. Nano Letters, 15 (2015), p. 2168.