Exploring the Multipurpose Potentials of Functionalised Nanoparticles in Image Registration and Drug Delivery with High Resolution Correlative Fluorescence and X-ray Microscopy at Cryogenic Temperatures
- Abstract number
- 56
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.56
- Corresponding Email
- [email protected]
- Session
- Multimodal Microscopy
- Authors
- Amy Watts (1), Dr Archana Jadhav (1), Dr Kamal Nahas (1), Dr Claire Pizzey (2), Dr Maria Harkiolaki (1), Dr Chidinma Okolo (1)
- Affiliations
-
1. Beamline B24, Diamond Light Source
2. Industrial Liaison Unit, Diamond Light Source
- Keywords
Nanoparticles, Soft X-ray Tomography, Structured Illumination Microscopy, Drug Delivery, Correlated Microscopy,
- Abstract text
Up until now, nanometer-sized gold nanoparticle beads have been used as add-on features on cells’ external environment for use in alignment of image projections (tilt series) and reconstruction of these projections into three-dimensional volumes - tomograms. However, tilt series alignment, tomographic reconstruction and precise matching of fluorescence and X-ray features from different microscopes can be improved by facilitating the internalisation of these nanoparticles into the cells’ interior to enable their localisation at a varied length, breadth and depth of the cell. We devised a strategy to achieve nanoparticle internalisation and simultaneously uncovered an insight into the mechanism of nanoparticle uptake. This research portends diverse biomedical implications in areas such as drug delivery and advancing the field of multimodal microscopy.
Beamline B24 is the cryogenic soft X-ray tomography (cryoSXT) beamline for the life sciences at the UK’s National Synchrotron facility (Diamond Light Source). The pipeline at B24 is a well-established synchrotron-based imaging method for capturing high definition, three-dimensional data from biological samples in their near-native state. To localise chemical information within the cellular landscape, structured illumination microscopy at cryogenic temperature (cryoSIM) is paired with cryoSXT. To automate the reconstruction of angular projections into tomograms, simplify same-sample and same-region data collection between two or more microscopes as well and aid 3D correlation, gold nanoparticles are usually deposited on the surface of the cells immediately prior to vitrification by plunge freezing. Presently, this is relatively effective, yet often, there are insufficient features within the depth (Z-plane) of the cell to guarantee successful processing or post-processing. Hence, the need for a basic-strategy to increase the palatability of nanometre-sized gold nanoparticles by cells. It is aimed that this strategy should also transcend fulfilling processing and post-processing needs and further help to provide three-dimensional positional markers for samples which will proceed from soft X-ray tomography to focused ion beam (FIB) electron microscopy.
We performed 3 separate experiments. One with beads coated in 10% bovine serum albumin (BSA), one with beads coated in 10% foetal bovine serum (FBS) and final test of our protocol on HeLa and U2OS produced through analysing these data. HeLa cells were cultured and seeded onto the carbon side of hydrophilised transmission electron microscope (TEM) grids. Gold nanoparticles (a mix of 100nm, 150nm, 200nm, 250nm) were prepared in either BSA or FBS, vortexed to homogenize and left at 4°C overnight for the proteins to coat the nanoparticles. The bead solution was then vortexed and incubated with each grid for 1hr, 3hr and 24hr. Cells were stained with fluorescent markers for F-Actin, lysosomes and mitochondria and fiducials were added to the surface of each grid before they were plunge-frozen into liquid nitrogen cooled liquid ethane. For the final test, we only used 200nm and 100nm, prepared separately in 10% BSA as described previously. We incubated the 100nm beads for 24hr and the 200nm for 1hr before adding 250nm fiducials to the surface and plunge-freezing. CryoSIM was used to collect high resolution fluorescent 3D datasets and these were reconstructed using SoftWoRX 6.5.2 (GE Healthcare). The transmission X-ray microscope was used to collect angular projections from (–60 to +60 degrees) of regions of interest and these tilt series data were reconstructed to tomograms by IMOD (version 4.9.2) (Kremer et al, 1996). CryoSIM and cryoSXT data were correlated using eC-CLEM (Paul-Gilloteaux et al., 2017) and analysed in Fiji (Schindelin et al., 2012).
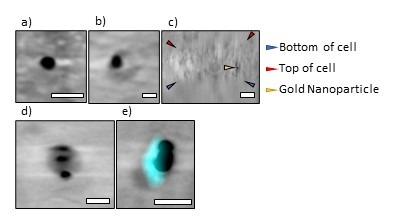
Through analysing orthogonal views of each tomogram and organelle fluorescent trackers, we observed that beads of all sizes were found within the interior of cell’s volume (Figure 1). The proportion of beads inside the cell was lower overall for beads coated in FBS than those coated in BSA. We also observed that these gold nanoparticles were inert and appeared not to trigger observable responses from the cells. These cells overall presented with healthy mitochondria and normal lysosomal activity in all experiments, further strengthening the hypothesis that these serum-coated gold beads were inert within the cellular ultrastructure. When testing the protocol in two different cell types, beads inside HeLa cells were in small vesicles that were tightly wrapped around each bead whereas U2OS cells had multiple beads in large vesicles (400 - 700nm). It remains unclear what these structures exactly are, hence, the need for further investigation. In certain conditions, correlation of cryoSIM and cryoSXT datasets portrays the colocalisation of gold nanoparticles within vesicles that stained positively for lysosomes (Figure 1), suggesting that internalisation and clearance mechanisms are dependent on nanoparticle size and cell line.
This work has successfully delivered internalisation of nanometer-sized nanoparticles; however, further experiments will aim to explain the mechanisms of internalisation of these nanoparticles. Beyond microscopy technique development, in the future, this study will drive towards using nanoparticles as vehicles for the targeted delivery of molecules within specific areas of the cell.
Figure 1: Representative data obtained from the strategic incorporation of functionalised nanoparticles to cancer cell lines. These 100nm beads have been coated in BSA and incubated with cells for 24hrs (a) 100nm gold fiducial outside a HeLa cell (b) 100nm gold fiducial inside a HeLa cell (c) an orthogonal (ZY) view of the same bead shown in (b) (d) 3 × 100nm gold beads inside a U2OS cell (e) correlated data showing co-localisation between the beads and lysosomes (cyan fluorescence) in U2OS cells. Scale bars (a) 200nm (b) 200nm (c) 500nm (d) 200nm (e) 200nm