From molecular-scale electrical double-layer structure to 3D nano-rheology properties of solid electrolyte interphase
- Abstract number
- 442
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.442
- Corresponding Email
- [email protected]
- Session
- Nanoscale Science of Materials for Energy Storage and Generation
- Authors
- Dr Yue Chen (1, 3, 4), Sergio Gonzalez-Munoz (1, 4), Wenkai Wu (2), Prof. Oleg Kolosov (1, 4)
- Affiliations
-
1. Lancaster University
2. Swansea University
3. Fujian Normal University
4. The Faraday Institution
- Keywords
Solid-state interphase, 3D-AFM, Electrical double layer; battery
- Abstract text
The solid electrolyte interphase (SEI), a passivation layer formed on the battery electrode-electrolyte interface [1], defines fundamental battery properties - its capacity, cycle stability, and safety. While understanding the SEI formation holds keys to these, such studies are complicated by the diversity of interlinked surface reactions and complex nanoarchitecture of the anode active material and electrical double layer (EDL) [2]. Such nanoarchitecture predetermines the electrolyte supramolecular interactions, electrical charge, and ion transport, therefore, dominating the initial SEI formation. To date, the real space molecular arrangements of electrolyte solvents/anions inside the EDL and their effects on the SEI formation remain elusive [3].
In this work, we resolve this complex puzzle, using a novel solid-liquid interface characterization tool with a nanoscale spatial resolution for accessing the whole evolution process from initial molecular-scale EDL structures, toward nanoscale 3D SEI structures. We introduce in-situ electrochemical 3D nanorheology microscopy (3D-NRM) [4] combined with magnetic excitation molecular-level solvation force spectroscopy and molecular dynamics simulations to explore a matrix of two morphologically dissimilar but chemically identical surfaces of typical carbon electrode material (basal and edge graphene planes) and different solvent-electrolyte systems (strong and weakly solvating electrolytes, as well as ionic liquid electrolyte). These approaches allowed us to get direct insight into the atomistic pictures for the underlying influence of cation’s intercalation and solvation structures on the initial SEI formation.
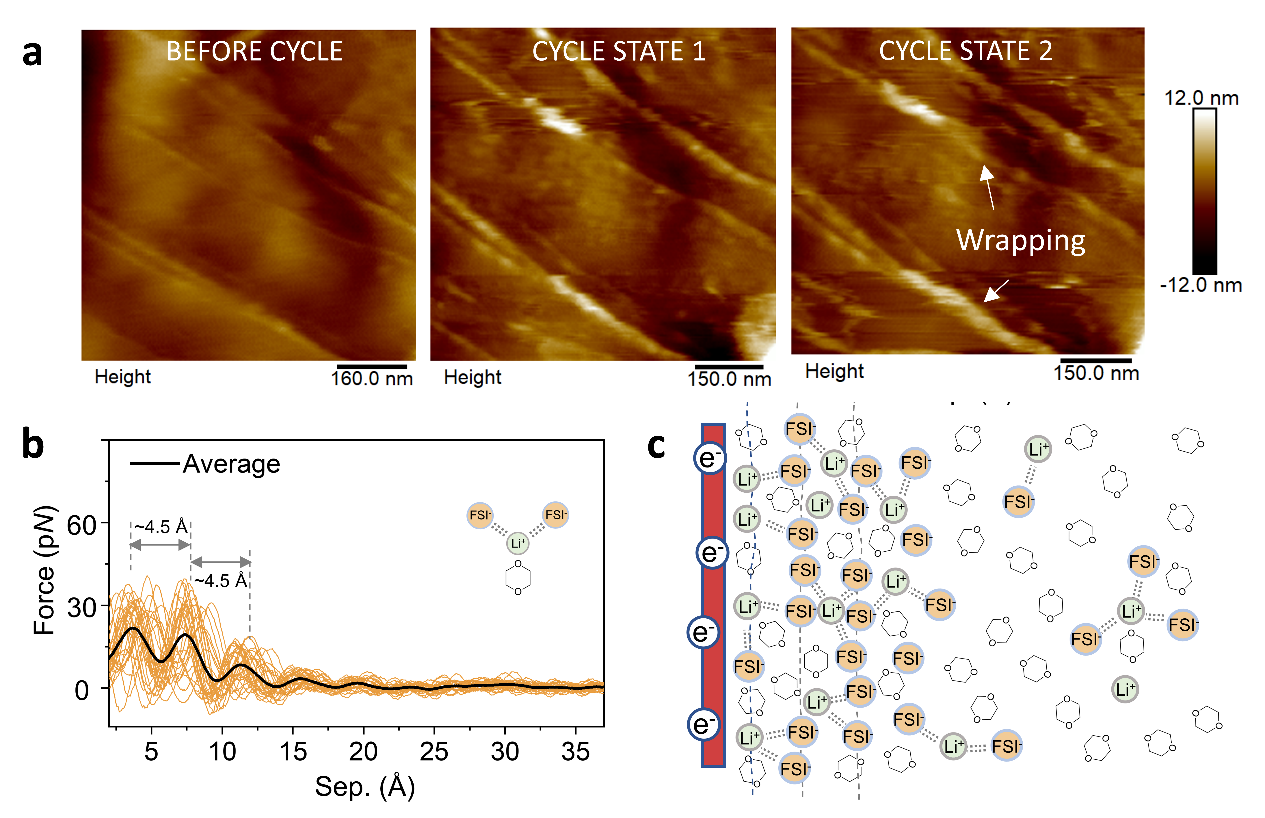
Figure 1 (a) SEI formation on graphite atomic step in EC-based electrolyte, (b) Surface force spectroscopy of graphite and 1 M LiFSI in 1,4 Dioxane electrolyte interface, (c) The sketch of nanoscale EDL model derived from the force spectroscopy.
We found that in the traditional ethylene carbonate (EC) based electrolyte, the strong coordination and co-intercalation of EC solvent dominate the electrolyte decomposition, causing the “swelling” of graphite carbon edge planes and thick SEI accumulations (Figure 1a), confirming that SEI formation is graphite crystal plane dependent. At the same time, in the weakly solvation and ionic liquid electrolytes, where the first cation solvation sheath is occupied by the fluorine-rich anion, the negatively polarized electrode surface builds up a unique and ordered EDL structure (Figures 1b, c) resulting in the preferential reductive decomposition of the anion and creating a highly sought after robust SEI layer. We also observe that the solvent/anion coordination structures with lithium/sodium cation within EDL vary from the bulk electrolyte, modifying the HOMO level of the electrolyte and affecting it electrochemical stability. Our understanding of these key interfacial structural factors in SEI formation allows targeting an electrochemically and mechanically robust surface passivation layer and guiding the development of efficient and safe rechargeable batteries.
- References
References:
1. Liu, T., et al., In situ quantification of interphasial chemistry in Li-ion battery. Nat Nanotechnol, 2019. 14(1): p. 50-56.
2. Rakov, D.A., et al., Engineering high-energy-density sodium battery anodes for improved cycling with superconcentrated ionic-liquid electrolytes. Nat Mater, 2020.
3. Li, C.-Y., et al., Unconventional interfacial water structure of highly concentrated aqueous electrolytes at negative electrode polarizations. Nature Communications, 2022. 13(1).
4. Chen, Y. et al, Controlling Interfacial Reduction Kinetics and Suppressing Electrochemical Oscillations in Li4Ti5O12 Thin-Film Anodes, Advanced Functional Materials 2021, 31 (43), 2105354.