Live super-resolution imaging of a multi-protein complex deep within the plant root tissue permits investigation of both slow and fast dynamics.
- Abstract number
- 457
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.457
- Corresponding Email
- [email protected]
- Session
- New and Emerging Concepts in Microscopy
- Authors
- Dr Raymond Wightman (1)
- Affiliations
-
1. Sainsbury Laboratory, University of Cambridge
- Keywords
Confocal, plant, root, xylem, cellulose synthase, confocal, Airyscan, Golgi, cytoskeleton
- Abstract text
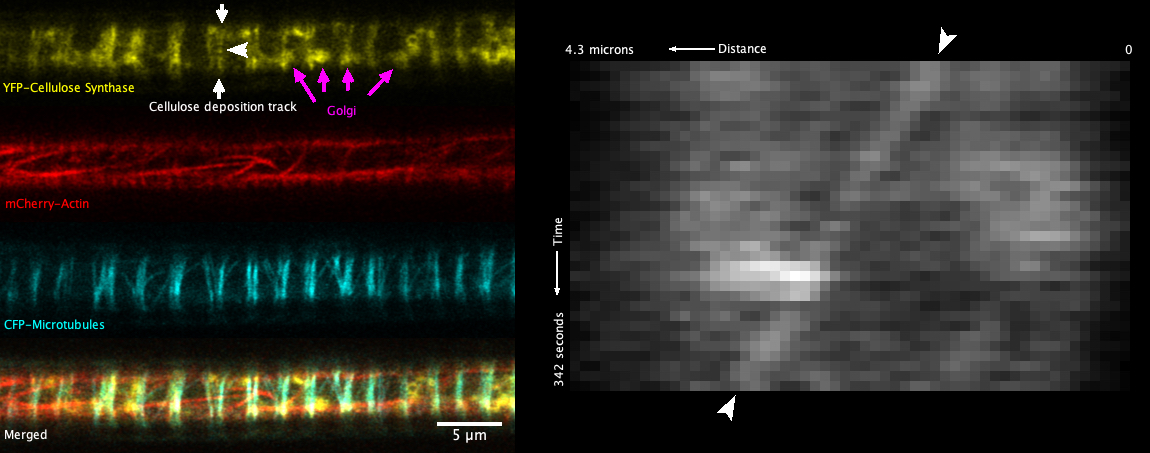
The cellulose synthase complex is a multi-protein complex that sits within the plasma membrane and synthesises and deposits cellulose directly to the plant extracellular matrix, termed the primary cell wall. In woody tissue, additional strength is given by the secondary cell wall, a reinforced composite consisting mainly of cellulose plus other components. One of the best known wood-cell types are the xylem vessels which are long, thin cylindrical vessels that transport water upwards. Developing vessels can be observed by fluorescence microscopy near the root tip. These vessels, termed protoxylem vessels, make their walls in hoops and spiral patterns along the 6 micron-wide cylindrical cell. The cellulose synthase complex can be observed in (bright) Golgi within the cell and in feint bands representing sites of secondary wall formation at the cell surface. Live imaging of developing vessels is challenging as (i) the vessels run along the centre of the root, requiring imaging at depth (ii) strong actin-driven cytoplasmic streaming sends the numerous bright Golgi rapidly along the length of the cell, obscuring events that are happening at the cell surface (iii) no particles representing surface cellulose synthase complexes have thus far been observed in non-epidermal tissue.
I have applied confocal airyscanning microscopy to address the problem of imaging surface cellulose synthases in the vessels in planta. Timelapse imaging at sub-second intervals has allowed me to resolve particles of complexes as they move beneath the sites of secondary wall formation, with velocities of nanometres per minute, against a backdrop of high speed intracellular dynamics measured in microns per second (Figure 1). The data confirm that the complexes move in concert as they deposit the cellulose, possibly arranged in arrays of individual particles. The data also give an insight in to the fast intracellular dynamics that result in delivery and replenishment of complexes to the cell surface where the force of cytoplasmic streaming reveals a close interaction between the Golgi and plasma membrane. The challenge now is to record these events in 3D and multiple channels while keeping the time resolution to below 1 second which, in turn, will allow tracking of complexes from formation in the Golgi, through delivery to the cell surface and to movement as they polymerise and deposit the cellulose to the wall.
Fig. 1 - A developing xylem vessel from a live root showing cellulose synthase, actin and microtubules (left panel) and a kymograph of a slow moving cellulose synthase particle (right panel). The vessel runs along the centre of the root, requiring super-resolution at depth. An example of a plasma membrane track of slowly moving cellulose synthase particles is shown by white arrows. The white arrowhead points to a single particle for which movement is shown as the slanted line in the kymograph. Examples of fast intracellular Golgi involved in delivery of cellulose synthases are shown by magenta arrows.
- References