Magnesium nanoparticles for metal enhanced fluorescence
- Abstract number
- 438
- Corresponding Email
- [email protected]
- Authors
- Dr Christina Boukouvala (1), Dr Vladimir Lomonosov (1), Prof Emilie Ringe (1)
- Affiliations
-
1. University of Cambridge
- Keywords
Metal enhanced fluorescence, magnesium nanoparticles, plasmonics, correlated single particle microscopy and spectrosopy
- Abstract text
Summary
Mg nanoparticles (NPs) are investigated as a platform for metal enhanced fluorescence (MEF). More specifically, the influence of metallic Mg NPs on the fluorescence emission intensity and lifetime of fluorescein isothiocyanate (FiTC) is studied in bulk and single particle level.
Introduction
Magnesium NPs have recently attracted attention for plasmonic applications owing to Mg’s broad plasmonic response (UV to IR), its biocompatibility and abundance on Earth’s crust, as well as its, unique among plasmonic metals, crystallography that gives rise to a variety of NP shapes [1]. While the optical response of Mg has previously been characterised both experimentally and numerically [2,3], Mg NPs’ performance in enhanced spectroscopies, such as MEF, a hallmark of plasmonic activity, is yet unexplored. Mg is appealing for MEF as its broad resonance wavelength range allows it to be matched optimally with a range of fluorophores.
Aim and objectives
This work aims to demonstrate that plasmon-induced local fields in Mg lead to spectroscopic enhancement in MEF. The first objective is to investigate the effects of a SiO2 spacer-shell of varying thickness on MEF signals akin to what has been done for other plasmonic metals [4]. The second objective is to determine the MEF signal enhancement by comparing the fluorescence intensity of metallic plasmonic Mg NPs and fully oxidized, non-plasmonic structures.
Materials and methods
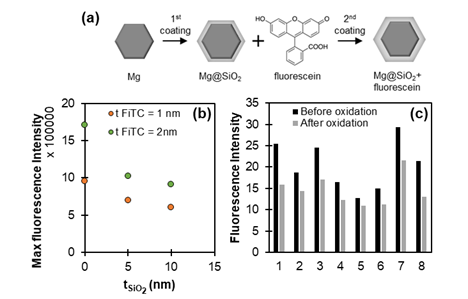
Mg NPs, exhibiting a self-limiting thin MgO layer (~5 nm), were synthesised by the reduction of an organic Mg reagent [5] and coated with a SiO2 silica shell of varying thickness using a modified Stöber method. After purification, the coating process is repeated with an additional silanised version of FiTC which is cross-linked inside the 2nd thin silica shell (Figure 1a). Fluorescence intensity and lifetime measurements are perfomed using the NP suspension in liquid form (spectrofluorimetry and time-correlated single photon counting measurements) or after they are left to dry on a glass substrate for dry/single-particle measurements (epifluorescence and fluorescence lifetime imaging measurements).
Results
Our results demonstrate that the fluorescence intensity of Mg@SiO2 NPs drops as the spacer (SiO2) thickness increases (Figure 1b). Assuming a ~5 nm MgO layer acting as an additional spacer to SiO2, the observed trend is consistent with that reported for Ag NPs where the maximum intensity was found for a spacer thickness of 5 nm [4]. The fluorescence intensity of dry Mg NPs was also found to decrease after exposure to water and subsequent NP oxidation (Figure 1c).
Figure 1. (a) Illustration of the sample preparation process. (b) Maximum fluorescence intensity for Mg@SiO2 NPs of varying SiO2 thickness and for two different thicknesses of the fluorescent shell. NPs are dispersed in ethanol. (c) Fluorescence intensity of 8 FiTC coated Mg NP and Mg NP aggregates (no SiO2 spacer) dried on a substrate before and after the oxidation of their metallic core.
Conclusions
These observations suggest that metallic Mg NPs can affect the fluorescence emission of FiTC. The results are to be corroborated with ongoing correlated intensity single particle measurements as well as ensemble and single particle lifetime measurements.
- References
[1] E. Ringe, J. Phys. Chem. C 124, 15665 (2020).
[2] J. S. Biggins, S. Yazdi, and E. Ringe, Nano Lett. 18, 3752 (2018).
[3] J. Asselin, C. Boukouvala, E. R. Hopper, et al., ACS Nano 14, 5968 (2020).
[4] J. Asselin, P. Legros, A. Grégoire, et al., Plasmonics 11, 1369 (2016).
[5] E. R. Hopper, T. M. R. Wayman, J. Asselin, et al., J. Phys. Chem. C 126, 563 (2022).