Minding the gap: Correlating structural and biochemical information of airborne London Underground pollution effects on airway cells.
- Abstract number
- 356
- Presentation Form
- Contributed Talk
- Corresponding Email
- [email protected]
- Session
- Public Health: The Impact of Microscopy
- Authors
- Miss Victoria Garcia Giner (2), Dr. Archana Jadhav (1), Dr. Sharon Mumby (3), Prof. Fan Chung (3), Prof. Ian Adcock (3), Dr. Maria Harkiolaki (1), Prof. Alexandra Porter (2)
- Affiliations
-
1. B24 Beamline, Diamond Light Source
2. Dept. Materials, Imperial College London
3. National Heart and Lung Institute, Imperial College London
- Keywords
Soft x-ray Microscopy
Structured Illumination Microscopy
Airborne Pollution
Cell culture
- Abstract text
The effects of urban air pollution to human health are a great concern to public health experts worldwide. Clinical studies overwhelmingly point to airborne pollution as a prevalent contributing factor in chronic cardiovascular and respiratory diseases in congested areas. The London Underground is a prime example of urban infrastructure where airborne pollution is known to be higher than above-ground levels and far beyond WHO recommended limits. Underground particulate matter (PM) is predominantly iron-based mixed with other redox-active metals (Mn, Cr, Ti) at lower concentrations alongside carbonaceous particles1. In vitro studies have demonstrated that PM exposure can trigger cellular oxidative stress2, inflammation and enhanced risk of infections. However, little is known about the mechanisms that effect toxicity and how cells interact with PM upon exposure. Currently, there is no correlated cell-level information about the effects of PM on airway cells metabolism, the structural damage to cellular organelles and the particular size and chemistry fractions of PM that cause this cellular damage.
In this study, cell culture models of airway cells have been exposed to fractionated London Underground pollution (PM0.1-2.5 µm and PM2.5-10µm) for 24h, and biological responses and ultrastructural remodelling were documented using Correlated Light And X-ray tomography (CLXT) at beamline B24 at the UK synchrotron Diamond Light Source3. Cryo Structured Illumination Microscopy (SIM) was used to obtain high resolution fluorescence information on PM-induced changes in mitochondrial membrane potential, cytoskeleton organisation and reactive oxygen species production. Cryo Soft X-ray Tomography (SXT) performed at the water window (500eV), was used to image the same cells and identify aberrant ultrastructural changes to cell membranes and organelles and the localisation of PM within them (Figure 1). Elemental near-edge absorption microscopy of exposed cells further provided information on the chemical composition of PM that found their way to cytoplasmic areas and linked to atypical ultrastructural presentations.
To generate a complete overview of London Underground PM effects in cellulo, it was necessary to develop a workflow that bridged multiple length scales of imaging. This work has moved from fluorescence microscopy (μm/mm-length scale to understand the biological response of stressed cells), to X-ray microscopy (nm-length scale to visualise the ultrastructural changes of cell organelles and PM location and composition). From a technical point of view, this new multiscale, multi-contrast microscopy workflow, is a promising tool for the nanotoxicological study of airborne pollution with biological matter. It can be translated into the use of primary airway cells from healthy and asthmatic volunteers, and more complex tissue-like cultures, such as Air-liquid-interfaces which mimics more closely the real human airways (preliminary results in place). Importantly, the relevance of this work could have an impact on the wider public as it allows us to identify the harmful PM fractions on the cellular level and can help create mitigating and therapeutic strategies to reduce human exposure.
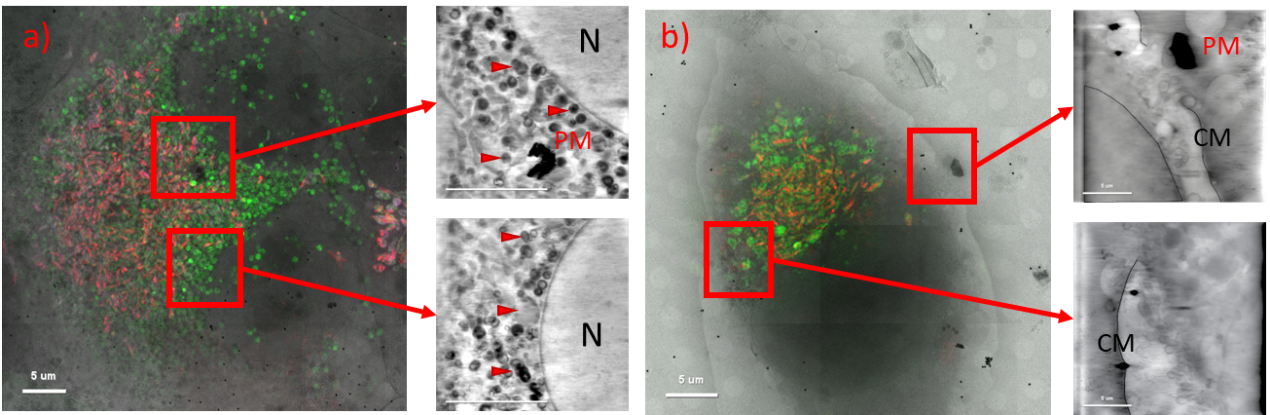
Figure 1. 2D cryo-SIM projection on cryo-SXT mosaics of A549 submerged cell cultures exposed to PM (0.1-2.5 µm) and slices of tomographic reconstruction of the areas highlighted. Super resolution fluorescence cryo-SIM data of mitochondria populations documented mitochondrial health and cryo-SXT assisted in understanding mitochondrial ultrastructural remodeling and PM presence within the cell. Fluorophores: JC-1 mitochondrial membrane potential (green-monomer-low membrane potential, red-aggregates- high membrane potential) and CellROX (magenta) Scale bars 5µm. a) PM 50 µg/mL dose. Large number of impaired small mitochondria (green). Insets: SXT tomogram slices of intracellular PM presence (top) surrounded by small mitochondria indicative of PM-induced fission events. b) PM 20 µg/mL dose. Insets; SXT tomogram of membrane disruption and excessive vesicle formation directed towards pollution particle (top).
- References
1. Kumar, P. et al. Characteristics of fine and ultrafine aerosols in the London underground. Sci. Total Environ. 858, 159315 (2023).
2. Lakhdar, R. et al. Lung toxicity of particulates and gaseous pollutants using ex-vivo airway epithelial cell culture systems. Environ. Pollut. 305, 119323 (2022).
3. Kounatidis, I. et al. 3D Correlative Cryo-Structured Illumination Fluorescence and Soft X-ray Microscopy Elucidates Reovirus Intracellular Release Pathway. Cell 182, 515-530.e17 (2020).