Observation of Oxygen Sublattice in the Structure Defects of Li1.2Ni0.13Mn0.54Co0.13O2
- Abstract number
- 95
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.95
- Corresponding Email
- [email protected]
- Session
- EMAG - Energy Materials
- Authors
- Dr Weixin Song (1), Prof Peter Nellist (1)
- Affiliations
-
1. University of Oxford
- Keywords
Li-rich cathode material; electron ptychography; stacking faults; oxygen shift
- Abstract text
Li-rich Li1.2Ni0.13Mn0.54Co0.13O2 (LRNMC) is a promising cathode material for next-generation Li-ion batteries because of the high specific capacity over 250 mAh g-1 delivered by the redox reaction of lattice O2- and transition metals (TMs) [1]. However, the voltages and capacities of LRNMC degrade on cycling and cause loss of energy densities. Unlocking the structural evolution of LRNMC in cycling is critical for accounting for the performance degradation. TM-layer stacking faults are widely-observed structural defects in LRNMC and vary with the TM migration in charge-discharge cycling [1]. The understanding of the evolution of such defects and their associations with degradation is yet insufficient.
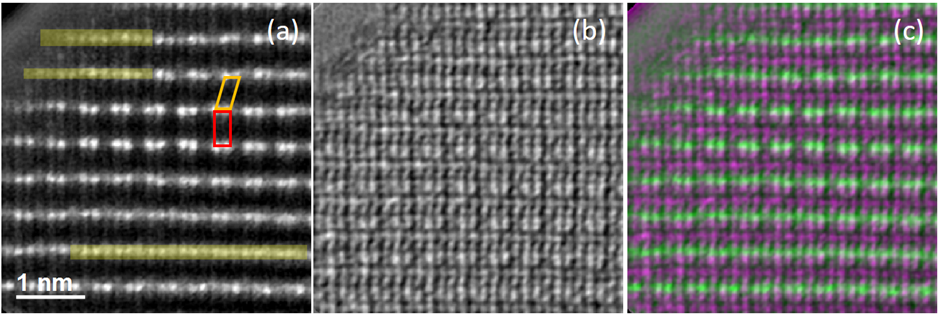
In this work, we performed simultaneous annular dark field (ADF) imaging and electron ptychography on LRNMC to show the TM and O sublattice at different charge-discharge cycling states, illuminating a picture of the evolution of stacking faults in cycling. Figure 1 displays the ADF, ptychographic phase image and the composite image of the prior two images of pristine LRNMC along the combined [100]&[110]&[1-10] zone axes. The dumbbell contrast in the ADF image is due to the Li+/Ni2+ and Co3+/Mn4+ cation ordering in the TM layers leading to a TM-TM ordering contrast in this projection. Rotational stacking faults of the TM layers are seen leading to domains that can be considered as being the [100] and [1-10] orientations of the TM layer labelled in red and orange, respectively. In contrast, the O sublattice associated with the faultily-stacked TM layers is seen as having an O3 stacking without faults. The TM layers also present disordered contrast in the ADF image which is labelled in yellow. Our 2D and 3D spectroscopy studies have shown an absence of elemental segregation in pristine LRNMC, together with the reported single-phase mapping results [2] supporting that LRNMC is a single monoclinic phase. The disordered contrast from TM layers can be due to inclined domain boundaries in projection. At the boundaries, the O sublattice remains in O3 stacking. In addition to the rotational stacking faults, we rarely observed another type of TM-layer stacking fault resulting from the layer shift by a vector of c/2 in the c direction. The O atoms show a clear shift with the faultily stacked TM layers, resulting from the strong TM-O octahedral coordination. The structure of pristine LRNMC is rather inhomogeneous and has various defects, which can impact on the O sublattice. Upon the charge-discharge cycling of LRNMC, TM migration occurs and changes the lengths of the ordering domains, resulting in an evolution of stacking faults and boundaries and the associated O sublattice.
Figure 1 Structural defects of pristine Li1.2Ni0.13Mn0.54Co0.13O2 simultaneously imaged by ADF and electron ptychography along the combined [100]&[110]&[1-10] zone axes. (a) ADF image. (b) Ptychographic phase image. (c) Composite image of the simultaneous ADF and ptychographic image. The green represents TMs and magenta light O and Li atoms. In ADF, the quadrilaterals in red and orange indicate the [100] and [1-10] domains, respectively. The yellow labels the TM-layer regions appearing disordering.
- References
[1] House, R.A., Rees, G.J., Pérez-Osorio, M.A., Marie, J.-J., Boivin, E., Robertson, A.W., Nag, A., Garcia-Fernandez, M., Zhou, K.-J., and Bruce, P.G. (2020). First-cycle voltage hysteresis in Li-rich 3d cathodes associated with molecular O2 trapped in the bulk. Nat. Energy 5, 777-785.
[2] Shukla, A.K., Ramasse, Q.M., Ophus, C., Kepaptsoglou, D.M., Hage, F.S., Gammer, C., Bowling, C., Gallegos, P.A.H., and Venkatachalam, S. (2018). Effect of composition on the structure of lithium- and manganese-rich transition metal oxides. Energ. Environ. Sci. 11, 830-840.
[3] The authors acknowledge John J. Marie and Peter G. Bruce, for providing the materials, and the use of characterization facilities within the David Cockayne Centre for Electron Microscopy, Department of Materials, University of Oxford, and in particular the Faraday Institution (FIRG016), the EPSRC (EP/K040375/1 “South of England Analytical Electron Microscope”) and additional instrument provision from the Henry Royce Institute (Grant reference EP/R010145/1).