Charting Cytoskeleton-Organelle Interplay in Living Cells Through High Resolution 3D Correlative Cryo-Imaging
- Abstract number
- 272
- Presentation Form
- Poster & Flash Talk
- DOI
- 10.22443/rms.mmc2023.272
- Corresponding Email
- [email protected]
- Session
- Multimodal Microscopy
- Authors
- Ivy Wang (3, 1), Dr. Peter Wing (3), Martijn van Nugteren (2), Dr. Michael Schwertner (2), Dr. Petros Ligoxygakis (3), Dr. Maria Harkiolaki (1)
- Affiliations
-
1. Diamond Light Source
2. Linkam
3. University of Oxford
- Keywords
Cytoskeleton, endocytosis, correlative imaging, X-ray tomography, structured illumination microscopy
- Abstract text
The early endosome serves as an essential sorting hub, enabling cells to process incoming signals and cues into responses. This complex process, ensuring molecules are sorted into the correct location at the right time, requires precise spatiotemporal regulation of numerous molecules. Recent studies have shown actin networks to be an increasingly important structure in the biogenesis of endocytic intermediates and their function in the sorting of internalized molecules. The ubiquity of actin in the cell underlines a need for a mechanism to polymerize actin in the appropriate cellular context. Currently, two proteins have been found to be essential in actin patch formation on the surface of endosomes. Annexin A2 is a member of a highly conserved family of calcium and phospholipid binding proteins while the WASP and Scar homolog (WASH) protein belongs to the Class I group of nucleation-promoting factors (NPFs). While well established to be key in linking actin dynamics with endosomal membrane dynamics, the mechanism with which Annexin A2 and WASH mediates precise endosomal membrane rearrangements required of the organelle through their actin network regulation abilities remains unclear. Additionally, with their similar involvement in actin regulation on the surface of endosomes, it will be important to clarify whether Annexin A2 works cooperatively, antagonistically or entirely independent of WASH. This insight will provide a more comprehensive view of the regulatory systems at play at the early endosome which will have important implications for our understanding of endosome physiology and consequently how the cell adapts to environmental challenges.
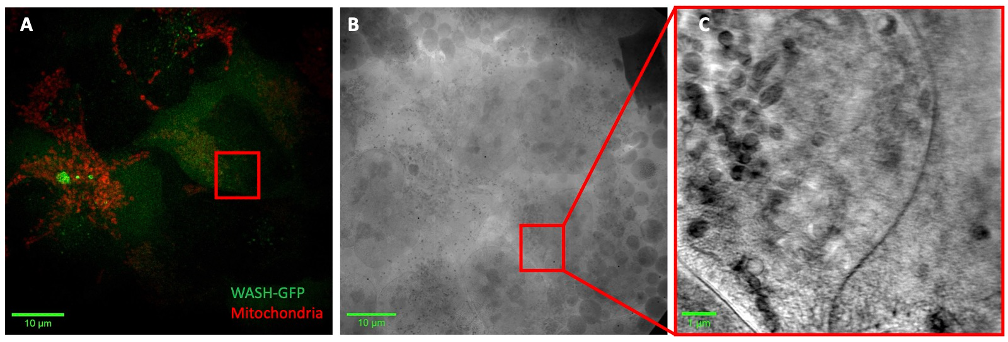
Previous imaging techniques have been limited in resolution and the ability to unambiguously trace proteins and actin filaments on subcellular organelles. Correlative cryo-structured illumination microscopy (cryoSIM) and cryo-soft X-ray tomography (cryoSXT), combines the precise localization information from fluorescence with high resolution ultrastructure information. To localize Annexin A2 and WASH, AnnexinA2-GFP has been introduced into U2OS cells using lentivirus transduction, while similarly a separate cell line was generated expressing WASH-GFP. U2OS-AnnexinA2-GFP and U2OS-WASH-GFP cells were grown on gold electron microscopy grids and stained with organelle dyes before being plunge frozen, preserving the cells in a near-native hydrated state. Super-resolution volumes of whole cells were collected by cryoSIM followed by collection of tilt series of regions of interest in the same cell based on the fluorescent information via cryoSXT. Tilt series were then reconstructed to generate 3D X-ray tomograms (Figure 1).
Figure 1. Figure 1. (A) Max intensity projection of structured illumination reconstructed volume of U2OS-WASH-GFP cells stained with mitochondrial dye (red). (B) X-ray mosaic of corresponding grid square in A with region of interest for tilt series collection (red box). (C) Slice through tomogram of region of interest in B.
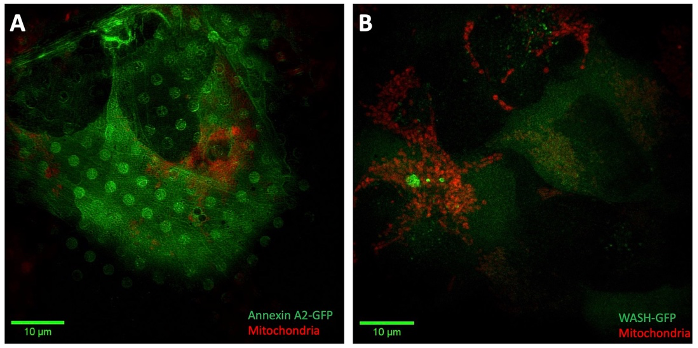
Preliminary fluorescence data corroborates successful expression of AnnexinA2-GFP and WASH-GFP in U2OS. Moreover, Annexin A2 and WASH have clear differences in their expression patterns within the cell suggesting diverging functionalities despite their mutual involvement in endosomal actin network organization. Annexin A2 appears to decorate filaments within the cell as well as the plasma membrane (Figure 2A). WASH has a prevalent cytoplasmic distribution with enrichment in puncta within the cell (Figure 2B).
Figure 2. (A) Max intensity projection of structured illumination reconstructed volume of U2OS-AnnexinA2-GFP cells stained with mitochondrial dye (red). (B) Max intensity projection of structured illumination reconstructed volume of U2OS-WASH-GFP cells stained with mitochondrial dye (red).
Correlation of fluorescent regions with their X-ray tomogram counterparts provides ultrastructural information that can clarify whether Annexin A2 and WASH elicit differences in actin network and endosomal membrane morphology. Moreover, the relative and precise localization of these actin organizing molecules to both actin and the endosome membranes can be characterized, providing a novel tool in the investigation of their role in endocytic sorting regulation. Furthermore, the response of Annexin A2 and WASH to environmental and pathogenic challenges will highlight how Annexin A2 and WASH may regulate the adaptation of the endocytic system to these challenges through their actin network organization activity. Characterizing this emerging role for actin in the endocytic system and the key molecules involved is essential in understanding cell signalling and how alterations in endosomal actin networks contribute to aberrant cell signalling.
- References
Duleh, S. N. & Welch, M. D. WASH and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton 67, 193–206 (2010).
Gomez, T. S. & Billadeau, D. D. A FAM21-Containing WASH Complex Regulates Retromer-Dependent Sorting. Developmental Cell 17, 699–711 (2009).
Morel, E., Parton, R. G. & Gruenberg, J. Annexin A2-Dependent Polymerization of Actin Mediates Endosome Biogenesis. Developmental Cell 16, 445–457 (2009).