Sample Preparation Methods for Simultaneous Imaging of Hard-Soft Bone Interfaces by Cryo-Electron Microscopy
- Abstract number
- 153
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.153
- Corresponding Email
- [email protected]
- Session
- FIB Applications & EM Sample Prep Techniques in Biological Sciences
- Authors
- Dr. Aekta Upadhyay (1), Dr. Angelique Galvani (2), Dr. Amina Merabet (1), Prof. Dr. Jean Le Bideau (1), Prof. Dr. Valerie Geoffroy (2), Dr. Patricia Abellan (1)
- Affiliations
-
1. Nantes University, CNRS, Jean Rouxel Institute of Materials of Nantes (IMN)
2. Nantes University, Oniris, Univ Angers, INSERM, Regenerative Medicine and Skeleton, RMeS
- Keywords
Cryogenic fixation, High Pressure Freezing, FIB-SEM, Bone Interface
- Abstract text
Interfaces between different biological tissues play a very important role in the biomechanics of native tissues and their study is crucial to understanding biological function [1]. Among them, the interfaces between hard and soft biological tissues, such as tooth-enamel interface [2], bone-tendon interface [3] and bone-cartilage interface [4] are particularly difficult to access due to the high mismatched behaviours in terms of bio (chemical), mechanical and physical properties [5]. Imaging hard-soft tissue interfaces has also become increasingly important in the field of tissue engineering. Among the different range of imaging modalities, electron microscopy is one of the most effective imaging tools to study the ultrastructure and elemental details at (sub)nanometric levels [1] and the only one capable of providing 3D information with a few nanometers spatial resolution from an area a few tens of micron in size by means of 3D focus ion beam (FIB) coupled with scanning electron microscopy (SEM).
Electron microscopy at cryogenic conditions allows the study of these details in near native conditions revealing much clearer information about the interface. Cryogenic fixation methods such as plunge freezing and high pressure freezing (HPF) are the most popular techniques to prepare the sample for imaging in electron microscopy. Both of these techniques reveal the structural details in near native conditions and do not involve any chemical modifications in the samples. However, plunge freezing suffers from the limitation that the depth of vitrification of plunge frozen samples, and this is particularly true for dense samples, is only upto a few micrometers [6]. Therefore in order to access these interfaces with field of views larger than 5 µm and without introducing any artifacts from the formation of crystalline ice, a different cryofixation method must be used. HPF overcomes this limitation by allowing the samples to be vitrified to a depth of upto 200 µm [7]. In this context, particularly challenging is the preparation of samples for characterization of hard-soft interfaces, for which cryo fixation require adapted methods and optimised techniques.
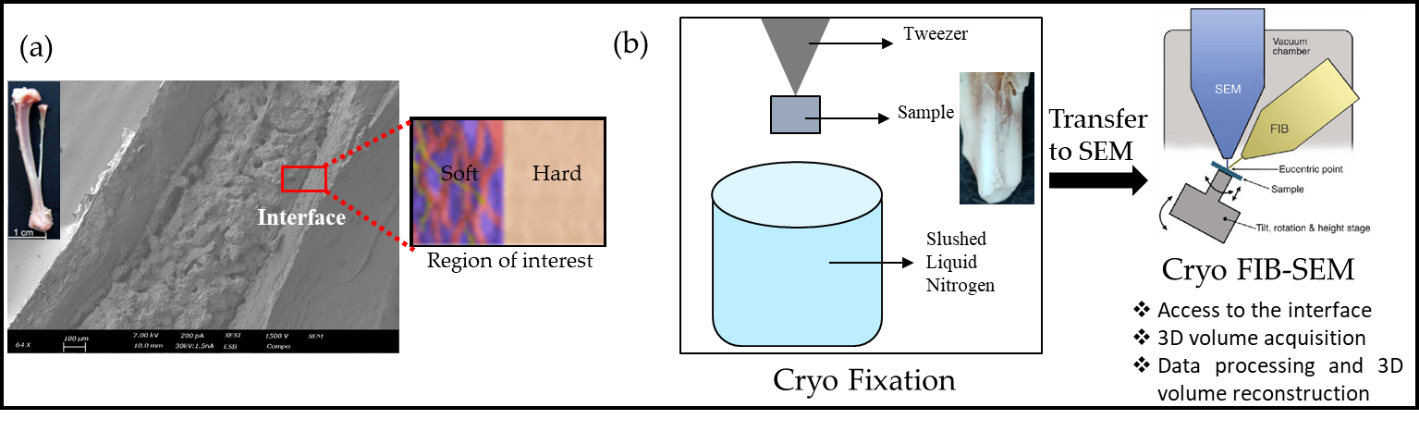
For our studies, we have chosen to develop the suitable sample preparation method for imaging the interface between cortical mineralized bone and not yet mineralized bone matrix (see schematic in Figure 1a) as a model system in cryo electron microscopy. In all our studies, the front legs of two months old wild mice were dissected and chemically-fixed immediately, to preserve the near native hydrated state of tissues.
In this presentation, various challenges and their solutions for sample preparation by cryo fixation (plunge freezing and HPF) will be discussed. In addition to this, we will also cover: (a) ways to access the interface and specific sites of interest within the 3D volume, (b) methodologies to acquire such high contrasting images with high spatial resolution while accounting for their high sensitivity to the electron-beam and charging effects and (c) acquisition and reconstruction of 3D volumes by 3D cryo-FIB/SEM (see Figure 1b).
Figure 1: (a) Diagram showing the area of interest (hard-soft interface) for the bone sample, (b) General workflow showing the cryo-fixation and transfer of sample to FIB-SEM.
ACKNOWLEDGEMENT
The authors would like to thank the financial support by the French National Research Agency (ANR) through the project JCJC VINCI (with reference ANR-20-CE11-0009).
- References
- Bannerman, A.; Paxton, J.Z. and Grover, L.M. Biotechnol. Lett. 36, 403-414 (2014).
- Reyes-Gasga, J.; Martinez-Pineiro, E.L. and Bres, E.F. J. Microsc. 248, 102-109 (2012).
- Spalazzi J.P.; Doty S.B.;Moffat K.L.; Levine, W.N. and Lu, H.H. Tissue Eng. 12, 497-3508 (2006).
- Khanarian, N.T.; Jiang, J.; Wan, L.Q.; Mow, V.C. and Lu, H.H. Tissue Eng. 18, 533-545 (2012).
- Fang, Y.; Yang, X.; Lin, Y.; Shi, J.; Prominski, A.; Clayton, C.; Ostroff, E. and Tian, B. Chem. Rev. 122, 5233–5276 (2022)
- Blancard, C. and Salin, B. J. Vis. Exp. 123, e54874 (2017)
- Kach, A. in “High Pressure Freezing” https://www.zmb.uzh.ch/static/bio407/assets/Bio407_HPF_2013.pdf