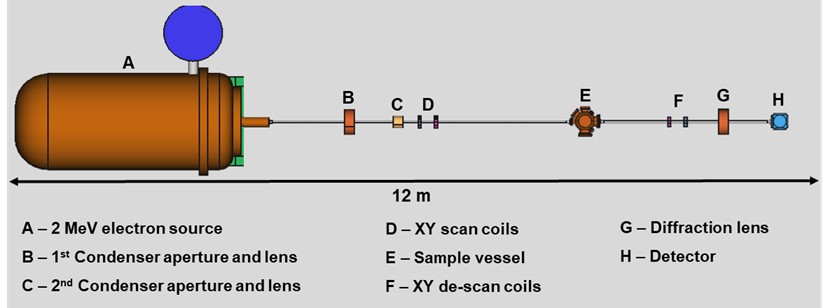
The High-energy Electron Xtallography Instrument: a tool for macromolecular structure determination
- Abstract number
- 140
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.140
- Corresponding Email
- [email protected]
- Session
- EMAG - High Resolution Structural Analysis
- Authors
- Dr Pedro Nunes (1), Graham Duller (1), Richard Littlewood (1), Mark Lunnon (1), Dr Gwyndaf Evans (1), Dr Alistair Siebert (1)
- Affiliations
-
1. Diamond Light Source
- Keywords
electron diffraction, three-dimensional electron diffraction, microED, macromolecular crystallography
- Abstract text
Funded by the Wellcome Trust “Electrifying Life Sciences” grant, the High-energy Electron Xtallography (HeXI) project aims to build an electron diffraction optimized instrument to investigate the use of Mega-electron-volt (MeV) electrons for macromolecular structure determination. This instrument will leverage the increased penetration of MeV electrons to bridge the crystal size gap between electrons and X-ray scattering and determine structures from crystals with sizes ranging between 300 nm and 3 μm.
The HeXI instrument will enable rapid high-resolution structure determination from crystal sizes which are inaccessible to both conventional electron diffraction in transmission electron microscopes (TEM) which requires sample sizes below 300 nm, and X-ray scattering methods which need crystals over 1 μm in size. This novel instrument will marry the unique sensitivity of electrons to structural information with the performance and fidelity of goniometry developed at Diamond for macromolecular crystallography beamlines. Users will be able to determine structures inaccessible to X-rays and additionally generate structural information complementary to that obtained using macromolecular X-ray crystallography (MX) as the Coulomb potential maps obtained by electron diffraction provide complementary information to electron density maps. For example, the intrinsic sensitivity of electron diffraction to hydrogen atoms has been used to identify of the position of hydrogen and hydrogen-bond networks in small peptides and proteins [1]. Moreover, electron diffraction Coulomb potential maps reveal the charge states of atoms which are particularly relevant in ligand binding studies and drug development [2]. .
The HeXI instrument at Diamond will offer data collection under three different modalities:
- Three-dimensional electron diffraction (3DED) of small molecules - Will enable the structures of small molecules to be determined from synthesis products directly, without further purification or crystallization, thus offering users a vial-to-structure workflow [3].
- Cryo-3DED of macromolecules – Will enable structure determination from frozen-hydrated protein crystals with sizes too small for MX beamlines and too large for 3DED on TEMs [4].
- SerialED – Will enable time-resolved (ms to μs) structure determination of macromolecules [5].
- References
- M. T. B. Clabbers et al., Journal of Structural Biology: X , 6 (2022) 100078
- M. D. Purdy et al., PNAS, 2018, 115 (52) 13258-13263
- C. G. Jones et al., ACS Cent. Sci., 4, 11 (2018) 1587–1592
- A. Lanza et al., IUCrJ, 6, (2019) 178–188
- R. Bucker et al., Nature Communications, 11, (2020) 996