Using cryo-S/TEM to identify changes in surface chemistry on food grade titanium dioxide particles suspended in cell culture media.p
- Abstract number
- 358
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.358
- Corresponding Email
- [email protected]
- Session
- EMAG - 3D & Tomographic Electron Microscopy
- Authors
- Teresa Roncal-Herrero (1), Martha Ilett (1), Sarah Ferris (1), Iqra Shah (1), Zabeada Aslam (1), Stuart Micklethwaite (1), Prof. Andy P. Brown (1)
- Affiliations
-
1. School of Chemical and Process Engineering, University of Leeds
- Keywords
Food-grade titanium dioxide particles, cryo-TEM, surface chemistry of nanoparticles
- Abstract text
Introduction
Titanium dioxide (TiO2) is an inorganic compound, primarily used in its particle form as a pigment or photocatalyst. It naturally occurs in three phases: anatase, rutile and brookite. Rutile and anatase phases have high refractive indices of 2.55 and 2.80 respectively, imparting performance as a white pigment ideal for use in paints, plastics, inks or food products [1]. TiO2 powder produced for use a food additive is referred to as E171 in the European Union but is no longer consider safe by the European Food Safety Authority (EFSA) since 2020 due to its potential genotoxic effects (damage to DNA) [2]. Whilst there is uncertainty surrounding E171 and the long-term effects of human exposure, accumulation of TiO2 particles has been found in human tissue, and studies have been conducted in mice, where damage has been caused to the brain, liver and kidneys [3]. Uptake of food grade (fg-)TiO2 nanoparticles has been observed in the Peyer’s patch in the small intestine of humans and mice, but it is not yet well understood how the particles cross the intestinal barrier into this tissue [4].
The microscopy work presented here on suspensions of particles in various aqueous media sheds light on how the surface chemistry of fg-TiO2 changes under conditions found in the small intestine (raised pH of ~ 7.5). Identifying surface precipitation of calcium and phosphorous based salts from the media provides insight into how fg-TiO2 might be transported into the Peyer’s patch of the small intestine.
Materials
Food-grade (fg-)TiO2 powder (AgResearch Limited) in the form of anatase was suspended in de-ionised (DI) water and cell culture media at physiological pH (~ 6.75). Three dispersions of 100 μg/ml TiO2 DI water and Dulbecco’s Modified Eagle’s Medium (DMEM) cell culture media at pH 6.8 and DMEM at pH 7.8 were prepared. Dry fg-TiO2 was analysed using X-Ray diffraction (Malvern Panalytical Empyrean X-Ray Diffractometer with a monochromatic copper Kα source (λ = 1.541 Å ). The surface of the nanoparticles was investigated using UHV X-ray photoelectron spectroscopy (XPS) using a Thermo Escalab 250 with PHOBIOS detector and monochromatic Al Kα source. Aliquots of the suspensions were analysed by Dynamic Light Scattering (DLS) using a Malvern ZetaSizer Nano ZS/ZSP. For microscopy, cryo-TEM samples were prepared by rapidly freezing 3 mL of aliquots on holed carbon films (EM resolution) using a FEI Mark IV Vitrobot© and transferred into a Gatan-914 cryo TEM holder at temperatures below -165oC. Analysis was carried on a FEI Titan Themis G2 equipped with a monochromator operating at 300 kV and fitted with 4 EDX detectors and a Gatan One-View CMOS camera at probe current < 100 pA. Some grids were plunge frozen and vacuum dried (PFVD) rather than being cryo-transferred and these were imaged in a Hitachi SU8230 SEM in STEM mode operated at 20 kV.
Results and Discussion
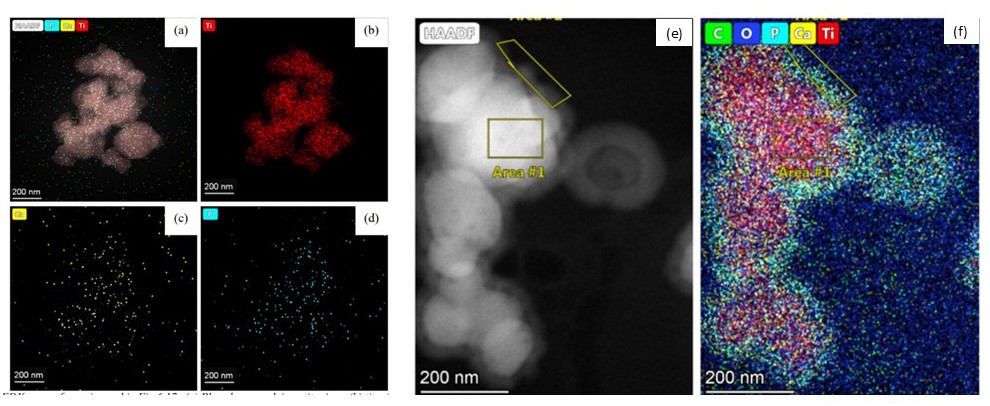
The raw powder was found to be pure anatase by XRD and Transmission Electron Microscopy (TEM) showed that 47% of the primary particles had a diameter of <100 nm. The presence of phosphorus on the particles was suggested by Energy Dispersive X-Ray spectroscopy and confirmed to be in the form of a surface phosphate by X-Ray Photoelectron Spectroscopy. When the fg-TiO2 is suspended in DI water DLS and PFVD-STEM show a stable dispersion of particle agglomerates a few 100 nm in size (Figure 1a). The water suspension has a negative zeta potential which we assume to be due to the phosphate coating on the particle surfaces. Similar agglomeration of particles was seen in a cell culture medium at pH 6.8 by PFVD-STEM as DLS results in cell culture media containing phenol-red dye used as a pH indicator were inconclusive. When the pH of the cell culture media is increased to 7.8 (to approximate the conditions within the human small intestine), cryo-TEM with EDX reveals that calcium and phosphorus ions from the media precipitate as an amorphous calcium phosphate coating on the particles (Figure 1b). The cryo-transfer process ensures the particles are preserved in the suspended state and eliminates the possibility of the precipitation of calcium phosphate as a drying artefact on the surface of particles.
Amorphous calcium phosphate particle uptake has been identified as a potential biological pathway used to chaperone bacteria and antigens to immune cells in the Peyer’s patch [5]. Cell culture media has similar calcium and phosphorous concentrations to gastric fluids and we show here that for such media, calcium phosphate will precipitate onto and coat fg-TiO2 when pH rises above 7 [6]. We suggest therefore that formation of a 20-50 nm thick calcium phosphate coating on fg-TiO2 in-vivo may facilitate the transport of the TiO2 from the intestine into the Peyer’s patch where its uptake has been observed.
Figure 1 a-d) STEM-EDX elemental maps of raw fg-TiO2 powders drop-cast on holey carbon films, note the limited calcium and phosphorous signal associated with these particles. e-f) cryo STEM-EDX elemental maps of fg-TiO2 powders suspended in cell culture media at pH7.8, note the 20-50 nm, amorphous, calcium and phosphate coating around these particles.
- References
1. Auer et al 2017. Pigments, Inorganic, 2. Ullmann, F. ed. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley.
2. Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E 171) (Text with EEA relevance). [Online]. [Accessed 2 May 2022]. Available from: https://eur-lex.europa.eu/
3. Winkler et al. 2018. Journal of Nanobiotechnology, 16(1), p.51
4. Riedle et al 2020. Small, 16(21), 2000486.
5. Powell et al. 2015. Nature Nanotechnology, 10(4), p.361–369.
6. Minekus et al. 2014. Good Funct., 5, 1113