What radiation does to water: the case of acidity under electron and X-ray exposure
- Abstract number
- 164
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.164
- Corresponding Email
- [email protected]
- Session
- EMAG - In-situ EM Techniques & Analysis
- Authors
- Andreas Körner (1), Birk Fritsch (1, 3), Dr. Thaïs Couasnon (4), Dr. Roberts Blukis (4), Prof. Liane Benning (4, 5), Dr. Michael P.M. Jank (2), Prof. Erdmann Spiecker (3), Dr. Andreas Hutzler (1)
- Affiliations
-
1. Forschungszentrum Jülich GmbH, Helmholtz Institute Erlangen-Nürnberg for Renewable Energy (IEK-11)
2. Fraunhofer Institute for Integrated Systems and Device Technology IISB
3. Friedrich-Alexander-Universität Erlangen-Nürnberg, Department of Materials Science and Engineering, Institute of Micro- and Nanostructure Research (IMN) and Center for Nanoanalysis and Electron Microscopy (CENEM)
4. GFZ German Research Center for Geosciences
5. Department of Earth Sciences, Freie Universität Berlin
- Keywords
- radiation chemistry
- liquid phase transmission electron microscopy
- X-rays
- irradiation
- kinetic reaction modeling
- Abstract text
We simulate how the concentrations of species, especially H+ and OH-, are influenced by irradiation with an electron beam or X-rays.
In situ or operando techniques such as liquid phase transmission electron microcopy or synchrotron-facilitated X-ray diffraction provide insights into fundamental material dynamics in liquid media. Changes to the local solution chemistry due to ionizing radiation can be modeled [1] but remain poorly understood.
Therefore, we follow a kinetic modeling approach to simulate radiolytic effects in aqueous solutions using AuRaCh [2]. We show that under irradiation the ion product of [H+] and [OH-] becomes decoupled from the autoprotolysis of water (see Fig. 1). A single parameter, namely pH, is then not sufficient to describe the acidity of the solution. Consequently, we introduce a new measure, the radiolytic acidity π* and radiolytic ion product Kw*, to adequately describe the system [3]. Our model predicts a compensation of initial concentration differences towards a neutralization of [H+] and [OH-] concentrations with increasing dose rate (Fig. 2). Furthermore, we show that by adding species that are pH irrelevant under standard conditions, depending on dose rate the acidity can be tailored to influence experimental conditions.
To conclude, we provide a kinetic model that allows to simulate various aqueous solutions enabling to estimate experimental conditions under irradiation with electrons or hard X-rays. The results are used to introduce a novel concept to describe acidity in irradiated solutions better and to predict the chemical environment under the influence of ionizing irradiation.
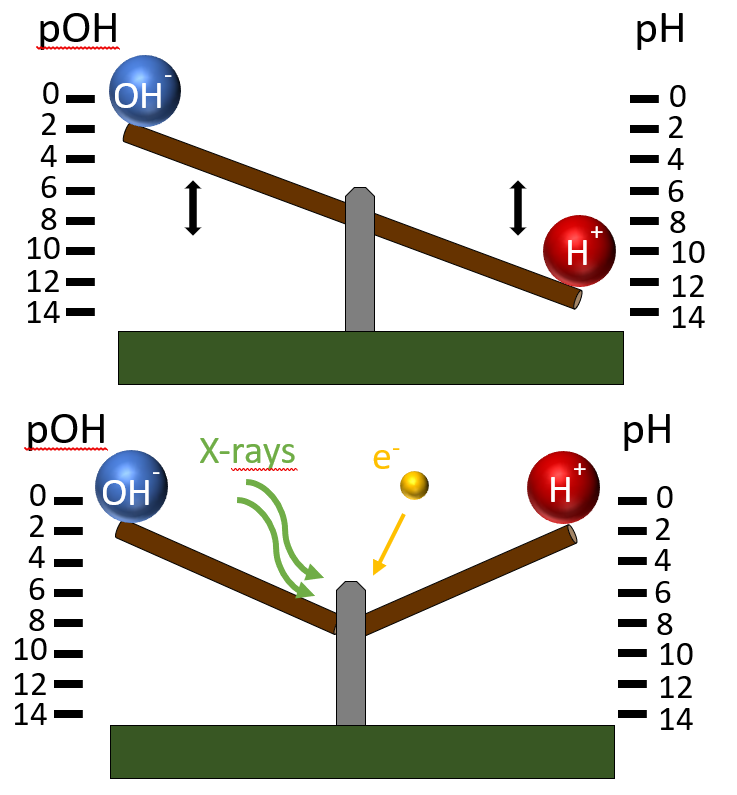
Fig. 1: The concentrations of [H+] and [OH-] are usually dependent on each other due to autoprotolysis (top). When the system is irradiated with ionizing radiation, this dependency is lost (bottom).
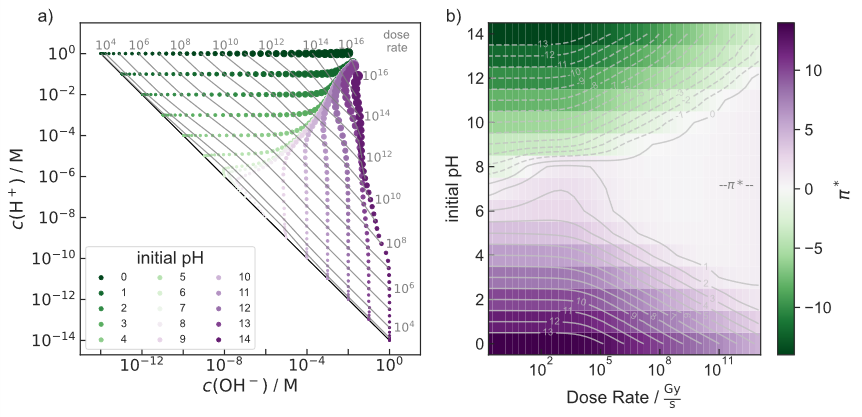
Fig. 2: Acid/base chemistry of pure, aerated water as a function of dose rate of an electron beam and the initial pH value. (a) Concentrations of H+ and OH− in the steady state. Each dot represents a simulation, while its size is a measure of the dose rate. Dose rate (grey numbers) is given in Gy · s−1 and indicated by contour lines. The black diagonal line corresponds to water under equilibrium conditions (KW = 10−14 M2) without irradiation. (b) π∗ (color map and grey contour lines) as function of initial pH and dose rate.
Acknowlegdements: A. Körner, B. Fritsch and A. Hutzler gratefully acknowledge financial support by the Federal Ministry of Education and Research (BMBF) of Germany in the programme H2Giga - StacIE. (Project identification number: 03HY103H). Financial support via the research training group GRK1896 “In situ microscopy with electrons, X-rays and scanning probes” is gratefully acknowledged. L.G. Benning acknowledges the support of the Helmholtz Recruiting Initiative (grant nro. I-044-16-01).- References
[1] N.M. Schneider, M.M. Norton, B.J. Mendel, J.M. Grogan, F.M. Ross, H.H. Bau, Electron–Water Interactions and Implications for Liquid Cell Electron Microscopy, J. Phys. Chem. C 118 (2014) 22373–22382. https://doi.org/10.1021/jp507400n.
[2] B. Fritsch, T.S. Zech, M.P. Bruns, A. Körner, S. Khadivianazar, M. Wu, N. Zargar Talebi, S. Virtanen, T. Unruh, M.P.M. Jank, E. Spiecker, A. Hutzler, Radiolysis‐Driven Evolution of Gold Nanostructures – Model Verification by Scale Bridging in situ Liquid‐Phase Transmission Electron Microscopy and X‐Ray Diffraction, Adv. Sci. 9 (2022) 2202803. https://doi.org/10.1002/advs.202202803.
[3] B. Fritsch, A. Körner, T. Couasnon, R. Blukis, M. Taherkhani, L.G. Benning, M.P.M. Jank, E. Spieker, A. Hutzler, Tailoring the Acidity of Liquid Media with Ionizing Radiation -- Rethinking the Acid-Base Correlation Beyond pH. Preprint, arXiv (2022) v1. https://doi.org/10.48550/arXiv.2209.05331.