ESEM as a Tool for Observations of Delicate Frozen Samples
- Abstract number
- 58
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2021.58
- Corresponding Email
- [email protected]
- Session
- Poster Session 4
- Authors
- Kamila Závacká (1), Martin Olbert (1), Vilém Neděla (1)
- Affiliations
-
1. Institute of Scientific Instruments of the Czech Academy of Science
- Keywords
ageing, ESEM, ice samples, thermodynamics
- Abstract text
The instrumental capabilities of observing frozen samples in electron microscopy are still not sufficient for visualizing unfixed, morphologically and chemically unmodified nonconductive samples in the environmentally relevant sub-zero temperatures. Here, we present new environmental scanning electron microscopy (ESEM) method which overcomes the technological deficiencies of the commercial microscopes in order to observe the unchanged morphology of the frozen aqueous sample at thermodynamic equilibrium.
The importance of research of frozen aqueous solutions lies in the still not fully understood chemical processes during freezing, which has a wide impact in the drug preparation in pharmaceutical industry [1] preservation of food [2] or description of atmospheric processes [3]. The low pressures (< 10-2 Pa) which must be applied in the electron microscope in order to avoid electron scattering [4] are unsuitable for observation of ice at environmentally relevant temperatures (above -50°C) because of its sublimation. Therefore, the environmental scanning electron microscopy (ESEM) shows to be the suitable tool for very sensitive observation of the frozen samples. However, the possibilities of commercial ESEM microscopes are usually limited to the usage of one type of working gas, minimal temperature of the Peltier stage (-20°C), unknown real temperature of the sample surface and unknown influence of the gas flowing around the sample. Our aim is to go beyond the possibilities of commercial microscope and provide a solution for trustworthy observation of static frozen samples at environmentally relevant temperatures.
The techniques of preparing frozen samples consisted of spray-freezing of ice spheres by spraying solution into a vessel containing liquid nitrogen (-196°C) (Figure 1) and immersing liquid sample into the liquid nitrogen (Figure 2). All of the samples were cooled outside the ESEM specimen chamber. The samples were observed in the non-commercial ESEM AQUASEM II equipped with a new Combined System for high-efficiency detection of Secondary and Backscattered Electrons (CSSBE) and self-designed cooling stage optimised for ice investigation. We used the mixture of two gases: water vapor for enabling the thermodynamic equilibrium with the frozen aqueous sample and an amplifying gas. Finally, (for sample in Figure 2) we intentionally disturbed the thermodynamic equilibrium to reveal the inner structure of the sample and by usage of sensitive ISEDS detector for secondary electrons we were able to capture fine morphological details of the frozen sample. The electron beam energy was 20 keV using the beam current of 125 pA.
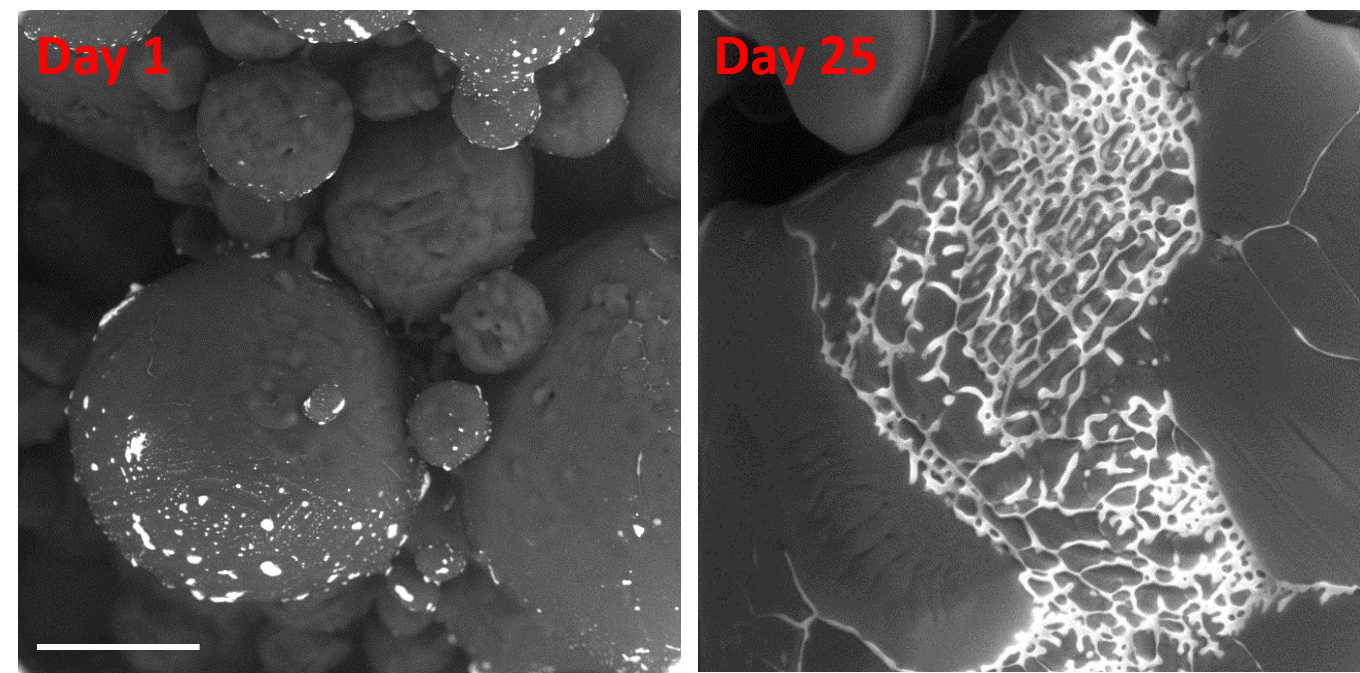
With our custom-modified microscope and new method, we can observe morphological modifications related to ageing of the samples, which are useful for the characterization of the influence of the storage conditions on the quality of the sample (e.g. food, drugs, old ice and snow on the poles). Figure 1 shows ageing of the ice spheres of frozen 0.06 M CsCl solution stored in the freezer at -20°C. The hexagonal ice can be seen as a dark grey and the freeze concentrated solution (FCS) as bright grey. We can clearly see the morphological changes of the ice spheres due to partial thawing of the hexagonal ice. Consequently, FCS in the morphologically changed ice spheres is flowing and mixing with the FCS contained in the neighboring ice spheres what can influence the change of physical-chemical properties of the FCS, e.g. change of pH, polarity, aggregation etc. Here, we are able to easily determine the dimensions of the sample, its surface morphology and location of the freeze concentrated solution.
Moreover, when optimal conditions meet (t < te(FCS), sufficiently high concentration of impurity, stable thermodynamic conditions in the specimen chamber) we can reveal the inner 3D structure of the sample. The possibility to cool the sample to -50 °C enables us to see the solid structure of the components that were present in the freeze concentrated solution (Figure 2 – a piece of frozen 0.5 M NaCl sample). During the solidification of the freeze concentrated solution all components freeze separately (brine: ice, salt) [5]. After sublimation of ice we revealed and observed thin long grain boundaries made of hydrohalite (NaCl · 2 H2O) crystals.
Our ESEM method and microscope modifications enable not only the in-situ dynamical [6], but also static, unchanging observations with the aim to show the true morphology of the inspected sample. Moreover, we provide a unique insight with high resolution inside the structure of the sample, which would not be possible without our special detector. [7]
Figure 1: ESEM observation of the frozen 0.06 M CsCl solution using the BSE detector of the combined system for high-efficiency detection of secondary and backscattered electrons (CSSBE). Bar scale corresponds to 100 µm.
Figure 2: ESEM image of frozen 0.5 M NaCl aqueous solution cooled rapidly in liquid nitrogen at -196 °C using the ISEDS detector for secondary electrons. Bar scale corresponds to 100 µm (left) and 10 µm (right). The fine structure of the FCS can be seen in right image which is a zoomed detail of the red square shown in the left image.
- References
[1] E. Y. Shalaev, F. Franks, Cryobiology, vol. 33 (1996), p. 14-26
[2] H. Kiani, D.W. Sun, Trends Food Sci. Technol., vol 22 (2011), p. 407-426
[3] T. Bartles-Rausch et al., Atmos. Chem. Phys., vol. 14 (2014), p. 1587-1633
[4] L. Reimer, “Scanning electron microscopy: physics of image formation and microanalysis.“ (Springer)
[5] N. Murase and F. Franks, Biophys. Chem., vol. 34 (1989), p. 293-300
[6] Ľ. Vetráková et al., International Journal of Pharmaceutics, vol. 585 (2020), p. 119448
[7] The project was supported by the Grant Agency of Czech Republic GA 19-08239S and GA 19-03909.