Imaging intracellular calcium-rich deposits using X-ray ptychographic tomography to reveal pathways of calcium transport in coccolithophore Emiliania huxleyi
- Abstract number
- 83
- Presentation Form
- Submitted Talk
- Corresponding Email
- [email protected]
- Session
- Stream 3: Volume Microscopy in Physical and Life Sciences
- Authors
- Mr Alexander Triccas (3), Dr Fabio Nudelman (3), Dr Tilman Grunewald (1), Dr Johannes Ihli (2), Dr Rachel Wood (3)
- Affiliations
-
1. European Synchrotron Radiation Facility
2. Paul Scherrer Institute
3. University of Edinburgh
- Keywords
Ptychography, tomography, calcium transport, biomineralization, coccolithophores, calcite, coccolith, electron microscopy
- Abstract text
SUMMARY
We used X-ray ptychography combined with computed tomography to identify and characterise calcium-rich deposits inside the cells of the dominant coccolithophore species Emiliania huxleyi. We analysed electron density of deposits and maturing calcite coccoliths, as well as the distribution and morphology of calcium-rich deposits, to improve our understanding of calcium transport in biomineralizing organisms.
INTRODUCTION
Coccolithophores are small marine phytoplankton dwelling in the surface ocean, which produce crystals of calcite (a polymorph of calcium carbonate) called coccoliths (A, B) in an intracellular compartment under very tight cellular control. Coccolithophores are essential in ocean biogeochemical cycles, providing the ballast to aid organic matter transport to the deep ocean to create the largest geological carbon sink in the ocean/atmosphere reservoir. They are also responsible for up to 40% of the global primary production and phytoplankton biomass, thus important for the maintenance of the marine food web.1 Despite the significance of coccolithophore biomineralization, we know very little about their mechanisms of calcification, and how these will be affected by anthropogenic change.
A key unanswered question in coccolith synthesis is how calcium and carbonate ions are transported to the site of mineralisation. Recent research has showed evidence of calcium transport via calcium-rich deposits in two species, Emiliania huxleyi and Pleurochrysis carterae.2,3 This avoids high cytosolic calcium concentration, which would be toxic to the cell. However, it is still unclear how calcium travels to the mineralisation site, as well as how the number, distribution and composition of deposits change over the course of calcification. This is partly due to the lack of time-resolved images in previous experiments.
X-ray ptychography is a coherent diffractive imaging method which, when combined with computed tomography, can be used to image large fields of view to produce an electron density map of a cell in 3D. This method offers the unique contribution of very high spatial cellular resolution combined with quantitative information on specimen composition that is obtained from electron density contrast. Using timepoints of cryogenically preserved coccolithophore cells at different stages of coccolith formation, we were able to characterise calcium-rich deposits in 3D using ptychographic tomography. Not only did this enable us to see how such deposits change in morphology and distribution over the course of coccolith synthesis, we could also determine how the electron density, related to calcium concentration, of deposits and maturing coccoliths changed over time. This allows us to make key deductions on the role that calcium-rich deposits play in the transport pathways of calcification.
MATERIALS AND METHODS
Cells from healthy cultures of E.huxleyi were decalcified and kept in low calcium (0.1 mM) seawater for 1 day to arrest coccolith formation. Calcification was induced by adding 0.1 M CaCl2.2H2O solution until the medium had reached [Ca2+] = 100 mM and the cells were then frozen under liquid ethane at different timepoints post-calcification; 0 hours (before calcification was induced), 1, 2 and 4 hours, and 24 hours (fully calcified cells). Cells were kept frozen under liquid nitrogen before being analysed using X-ray ptychography.
RESULTS AND DISCUSSION
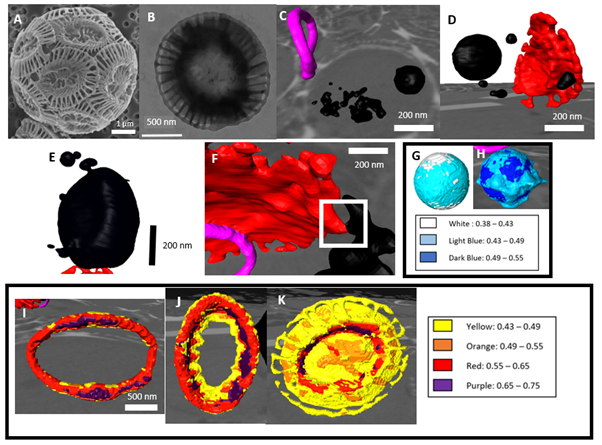
We were able to image cells at a resolution between 60-70 nm, which means that we could resolve individual intracellular compartments. Segmentation of intracellular material by electron density allowed for 3D reconstruction of coccoliths and calcium-rich deposits. We observed intracellular maturing coccoliths from initial stages (C) to fully developed (D), distinguished by the T-shape of the crystal units. We identified a number of intracellular compartments 20 – 500 nm in size with electron density values in the range of 0.43 – 0.49 ne Å-3, indicating that they were rich in calcium ions (as a reference, the electron density of ice is 0.31 and of organic components is ~0.43). Interestingly, the electron density of these deposits was higher in calcifying (H) than in non-calcifying cells (G), suggesting the uptake and accumulation of Ca2+ ions from the medium when in calcium-rich sea water. Furthermore, the size and distribution of the calcium-rich deposits varied, depending on whether the cells were calcifying or not. Non-calcifying cells had a single large deposit with small deposits nearby (E), whereas in calcifying cells deposits had a range of morphologies, distributions and frequencies (C-D). We were also able to observe deposits in contact with maturing coccoliths (F). These observations are novel in E.huxleyi, with only a single calcium-rich deposit observed in cells up to this point.2
We also analysed the electron density of intracellular forming and extracellular mature coccoliths. Interestingly, the electron-density of forming coccoliths was in the range of 0.55 – 0.75 around the centre rim (I, J), and in the range of 0.43 – 0.55, similar to that of the calcium-rich deposits (H), in the periphery (K). It is noteworthy that the electron density of mature coccoliths was still lower than 0.81, which is the theoretical value for calcite. These results suggest that there are changes in the composition and in the concentration of calcium as these structures are formed and mature.
Figures A-K: (A) SEM image of Emiliania huxleyi cell with coccoliths surrounding the cell. (B) TEM image of an extracellular coccolith. (C) 3D reconstruction of intracellular immature coccolith (pink) and calcium deposits (black). (D) 3D reconstruction showing calcium deposit in contact with a mature coccolith (red). (E) 3D reconstruction of a calcium-rich deposit in a non-calcifying cell. (F) Mature coccolith (red) with numerous calcium deposits. (G-H) 3D reconstruction of 200-nm calcium-rich deposit segmented by electron density in a non-calcifying (G) and calcifying (H) cell. Electron density values in ne Å-3 are shown in the legend. (I-K) 3D reconstruction of maturing intracellular coccoliths at different stages of growth. Electron density values in ne Å-3 are shown in the legend.
CONCLUSION
From our initial data analysis, we have shown calcium-deposits in E.huxleyi are more complex than previously observed. We have data to show calcium-rich deposits change when E.huxleyi begin calcifying. Our analysis of electron density on deposits and maturing coccoliths show values increasing as the structures mature, which will eventually be linked to changes in calcium concentrations. This data will help contribute to the wider understanding of calcium transport in biomineralizing organisms.
- References
1. Monteiro, Sci. Adv. 2, (2016).
2. Sviben, S. et al. Nat. Commun. 7, 1–9 (2016).
3. Gal, A. et al. Proc. Natl. Acad. Sci. U. S. A. 115, 11000–11005 (2018).