METEOR: an integrated top down cryo-CLEM imaging system
- Abstract number
- 59
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2021.59
- Corresponding Email
- [email protected]
- Session
- Poster Session 2
- Authors
- Marit Smeets (1), Anna Bieber (2), Cristina Capitanio (2), Oda Schioetz (2), Dr. Philipp Erdmann (2, 3), Prof. Juergen Plitzko (2)
- Affiliations
-
1. Delmic B.V.
2. Max Planck Institute of Biochemistry
3. Fondazione Human Technopole
- Keywords
cryo-CLEM, FIB/SEM, cryo-FIB/SEM, cryo-ET, cryo-lamella
- Abstract text
Here we present METEOR, a top down fluorescence light microscope (FLM) that enables integrated cryo correlative light and electron microscopy (cryo-CLEM) and enhances lamella sample yield for cryo electron tomography (cryo-ET).
Cryo-ET is emerging as a powerful technique to acquire high-resolution 3D structures such as intracellular organelles and protein complexes in their near-native cellular environment. However, the current sample preparation method can be challenging as it often requires cryo-focussed ion beam (FIB) milling to create an electron transparent lamella and correlative light microscopy to ensure the region of interest (ROI) is present in the lamella. METEOR reduces the number of transfer steps between microscopes, protecting the fragile sample from unnecessary contamination and damage. It also allows confirmation of the presence of the ROI in the lamella during and after FIB milling.
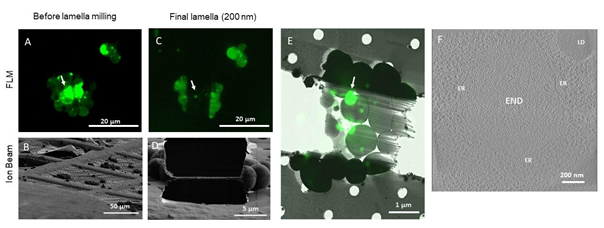
We demonstrated the abilities of METEOR by preparing targeted cryo-lamellae of Saccharomyces cerevisiae overexpressing Ede1-tagged with eGFP. Ede1 is a selective autophagy receptor and involved in the recruitment of proteins that form large condensates at the plasma membrane and within autophagic bodies1. We show that the fluorescence data from METEOR can be used to target these condensates and confirm the presence of the condensates during and after milling. By employing cryo-ET on FIB milled lamellae the Ede1 containing condensates were visualized with a high resolution by cryo-TEM (see Figure 1).
METEOR will increase the sample yield of specimens that need correlative microscopy to target protein structures and make correlative cryo-ET a more accessible and high-throughput technique.
Figure 1: Correlative cryo-FIB milling and cryo-ET workflow using METEOR, demonstrated on a sample of S. cerevisiae expressing eGFP-Ede1 A-D: Maximum intensity projections (MIPs) of fluorescence Z-stacks (A,C) and ion beam views (B,D) of a lamella before milling (A,B) and after fine milling (C,D). Fluorescence Z-stacks were acquired with excitation at 484 nm, an emission filter 525/30 nm and a z step size of 400 nm. E: Overlay of the final lamella MIP on the TEM lamella overview. Arrows in A, C and E indicate the targeted fluorescent punctum where the tomogram in (F) was taken. F: A 2D slice of a tomogram acquired on the indicated position on the lamella. The target structure, a phase separated END surrounded by fenestrated ER, can be clearly distinguished from the surrounding cytosol containing ribosomes. LD: lipid droplet
- References
1. Wilfling, F. et al. A Selective Autophagy Pathway for Phase-Separated Endocytic Protein Deposits. Molecular Cell 80, 1–15 (2020).