Novel experimentation chambers for improved and integrated live-cell imaging approaches.
- Abstract number
- 193
- Presentation Form
- Submitted Talk
- DOI
- 10.22443/rms.mmc2021.193
- Corresponding Email
- [email protected]
- Session
- Stream 6 (Frontiers): Quantifying Dynamic Movement in Living Cells
- Authors
- Dr. Alexander Lichius (1, 2)
- Affiliations
-
1. University of Innsbruck
2. inncellys GmbH
- Keywords
inncellys
experimentation chamber
live-cell imaging
fluorescence microscopy
live-cell physiology
3D-printing
customization
- Abstract text
Summary
inncellys experimentation chambers improve sample handling and application range of live-cell imaging approaches due to (1) standardised sample preparation, (2) injury- and stress-free sample mounting, (3) focus-stable imaging for up to 10 h continuously and (4) repeated investigation of the same sample for up to 72 h. The system is accessible to various optics, including bright-field, DIC, wide-field deconvolution and confocal fluorescence microscopy, and can be interchangeably used on upright and inverted microscopes. The basic design is readily customized to integrate different live-cell physiological measurements, such as light regulation, compound secretion and diffusion kinetics or volatile production. The prototype design for filamentous fungi is currently being adapted for plant-fungus-fungus co-cultivation to facilitate the study of inter-kingdom interactions.
Introduction
An experimental system that allowed live-cell imaging of cellular stress responses without introducing injury and mechanical stress artefacts due to sample preparation was commercially not available. Hence, we had to develop our own. The prototype chamber was designed for the examination of physico-chemical interactions between different species of filamentous fungi in the context of mycoparasitism. However, the functional principle is easily adaptable to other organism that require solid growth medium for live-cell imaging, such as plant seedlings or arbuscular mycorrhizal fungi. Current developments focus on novel solutions for plant-fungus-fungus co-cultivation to study molecular and cellular details of inter-kingdom interactions, such as between crop plant, fungal plant pathogen and mycoparasitic biocontrol fungus in the rhizosphere.
Methods
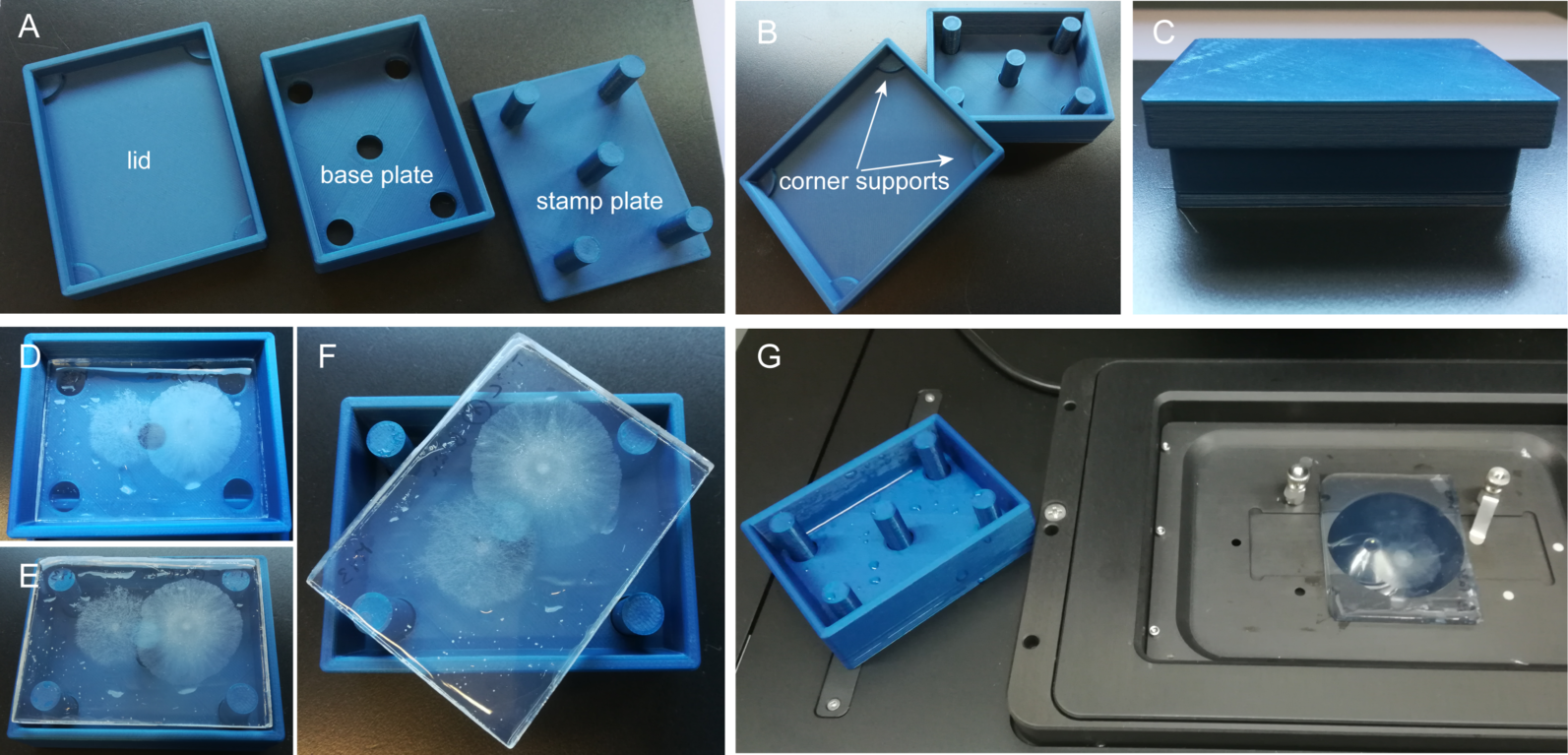
inncellys experimentation chambers are produced by CAD and 3D-printing. The inncelly basic chamber comprises a base plate and lid to form a closed, sterile yet ventilated incubation environment (Fig. 1). The basic shape is matched to the geometry of the inserted glass cover slip carrying the agar growth medium. The large medium reservoir allows focus-stable imaging for several hours continuously, or repeated imaging of the same sample for up to three days, provided the sample is returned to the chamber and intermittently incubated in a humid environment.
Figure 1. Construction and use of the inncelly basic chamber. (A) The chamber comprises a lid, a base plate and a stamp plate. (B) The lid guarantees sterile yet aerated incubation of the sample due to integrated corner supports. The stamp plate allows easy withdrawal of the sample. (C) Fully assembled chamber. (D) Chamber containing two fungal microcolonies growing on top of agar medium. (E) Withdrawal of the sample "sandwich" from the base plate with the help of the stamp plate. (F) The stamps raise over the rim of the base plate, allowing easy twisting and removal of the sample. (G) Rapid and easy transfer of the sample onto the microscope stage.
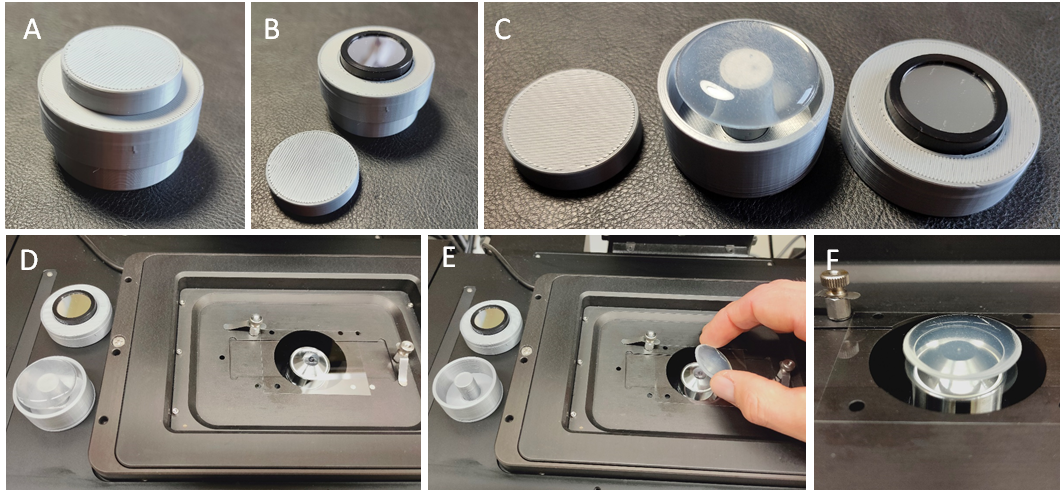
inncelly light chambers (Fig. 2) have filters with 10 nm bandwidth (in a range between 310-700 nm) fitted into the lid to allow precisely timed exposure to defined light qualities and quantities. Transfer of the sample onto the microscope stage is easy and very quick.
Figure 2. Construction and use of the inncelly light chamber. (A) Fully assembled inncelly light chamber for sterile, ventilated and dark incubation. (B) Removal of the filter cap uncovers the band pass filter and allows exposure to the desired light source. (C) The stamper plate fits through the bottom whole of the base plate to push out the sample. (D) The mobile chamber is easily transferred to the microscope and (E) rapidly mounted onto the microscope stage. (F) With pre-adjusted imaging settings the first image can be recorded within less than 20 second from removing the filter lid.
Results and Discussion
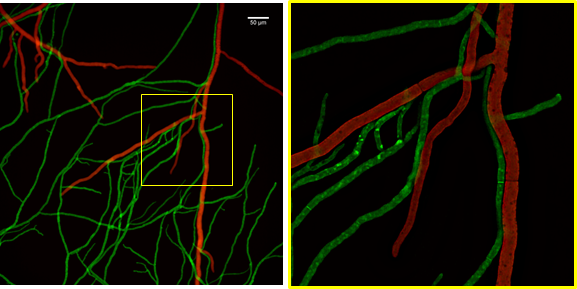
Application example I – Live-cell imaging of fungus-fungus interaction. The interaction between the mycoparasite Trichoderma atroviride and its phytopathogenic prey fungus Botrytis cinerea is a relatively slow process. The various stages from initial pre-contact chemical interaction to the physicochemical interaction between individual hyphae and eventual killing of the prey fungus stretches over 14-18 hours. By using inncelly basic chambers for sample preparation and mounting, up to 10 hours of the process can be covered in a continuous imaging session with highest temporal and spatial resolution (Fig. 3).
Figure 3. Live-cell fluorescence microscopy example recorded with the aid of the basic inncelly chamber. Mycoparasitic interaction between individual hyphae of two filamentous fungi. Trichoderma atroviride (green) and Botrytis cinerea (red), imaged with a Plan Apo 20x 0.75 N.A. objective on a wide-field fluorescence microscope (left) with subsequent deconvolution of the framed region (magnified on the right).
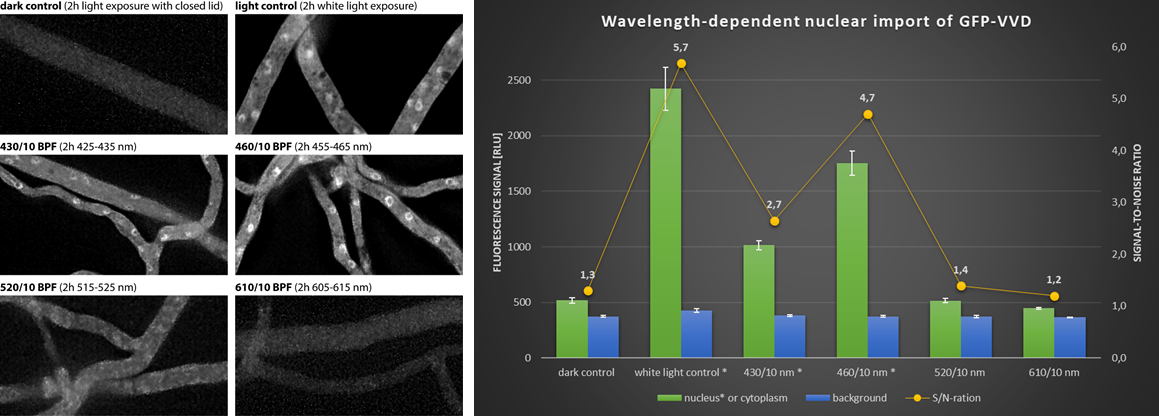
Application example II – Blue light dependent conidiogenesis. The development of pigmented asexual spores (conidia) in Trichoderma atroviride is controlled by light to guarantee that conidiogenesis occurs only above ground where wind distribution of the spores is possible. Using inncelly light chambers demonstrates that conidiogenesis is primarily triggered by blue light (Fig. 4).
Figure 4. Light dependent conidiogensis of T. atroviride. Pigmented conidia develop particularly under blue light. 460/10 nm induce full development, whereas 430/30 nm allow only initial differentiation. Green and red light have no inducing effect. Notably, the closed lid guaranties completely dark conditions under full illumination.
Application example III – Quantification of nuclear import of blue light photoreceptor. Vivid (VVD) is a photoreceptor in Neurospora crassa that transduces blue light signals into the nucleus. Nuclear import of GFP-VVD (Chen et al., 2010) occurs almost twice as efficiently in response to 455-465 nm than in response to 425-435 nm (Fig. 5). The quantification also shows that the inducing effect is additive under white light.
Figure 5. Light-induced nuclear import of VVD photoreceptor. N. crassa strain 826-1 (Pvvd::sgfp-vvd-v5; Chen et al., 2010) was pre-cultured for 16 h in darkness before being placed under cool white light for two hours to allow individual wavelength exposure through uncapped light filters (430/10 nm, 460/10 nm, 520/10 nm and 610/10 nm). Quantification of the nuclear signals allows to determine the inducing efficiency of the different wavelength ranges.
Conclusion
inncellys experimentation chambers offer versatile solutions to investigate cell biological processes with increased precision and the possibility to integrate various live-cell physiological measurements into the experimentation work flow. The production by CAD and 3D-printing allows rapid implementation and iterative optimisation of customized experimentation designs, and facilitates straight forward adaptation for different organisms and inter-kingdom interactions.
- References
References
Chen, C.-H., DeMay, B.S., Gladfelter, A.S., Dunlap, J.C., Loros, J.J., 2010. Physical interaction between VIVID and white collar complex regulates photoadaptation in Neurospora. PNAS 107, 16715–16720. DOI: 10.1073/pnas.1011190107 .