Quantifying Macrophage Phagocytosis of Bioparticles
- Abstract number
- 36
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2021.36
- Corresponding Email
- [email protected]
- Session
- Poster Session 3
- Authors
- Dr Meetal Solanki (1), Dr Rebecca Charlton (1), Dr Karen Hogg (2)
- Affiliations
-
1. Phasefocus
2. University of York
- Keywords
Phagocytosis
Fluorescence
Phase
QPI
Single-cell segmentation
Time-lapse imaging
- Abstract text
Phagocytosis is the process by which a cell (e.g. macrophage, neutrophil or dendritic cell) engulfs pathogenic or foreign particles, giving rise to an internal compartment called the phagosome. It is one of the main mechanisms of the innate immune system and a primary response to infection [1].
To understand the regulation of phagocytosis, and the impact that the cellular environment has on phagocytosis, particle engulfment must be quantified. Traditionally, this has been challenging but the use of fluorescent bioparticles combined with recent developments in real-time fluorescence microscopy has enabled measurement of total fluorescence intensity to quantify macrophage phagocytosis [2,3]. However, there are drawbacks with live fluorescence microscopy; for instance, phototoxicity is frequently encountered which can impair sample physiology, and even lead to cell death [4]. Furthermore, fluorescence microscopy analysis typically quantifies total fluorescence intensity which can be misleading and inhibits the study of population heterogeneity.
The aim of this study was to quantify phagocytosis of bioparticles by a RAW 264.7 macrophage-like cell line in a manner that both reduced phototoxic effects and reliably quantified cell fluorescence. In addition, as phagocytosis involves the actin-driven internalisation of particles, we sought to monitor the dose-response of the actin inhibitor cytochalasin D on phagocyte behaviour.
RAW 264.7 cells were seeded at 10,000 cells per well and allowed to adhere for approximately 24 hours. Cells were then incubated with pHrodo green E. coli bioparticles, which only fluoresce in the acidic environment of the phagosome and concentrations of cytochalasin D (10µM - 1nM). Label-free quantitative phase imaging, with a Livecyte Kinetic Cytometer [5], was used with intermittent fluorescence to automatically track cells over time and measure fluorescence periodically; this enabled investigation of phagocytosis whilst substantially reducing phototoxicity effects. The high contrast label-free images produced by Livecyte facilitated robust segmentation of cells and therefore phagocytosis activity could be quantified reliably by measuring individual cell fluorescence.
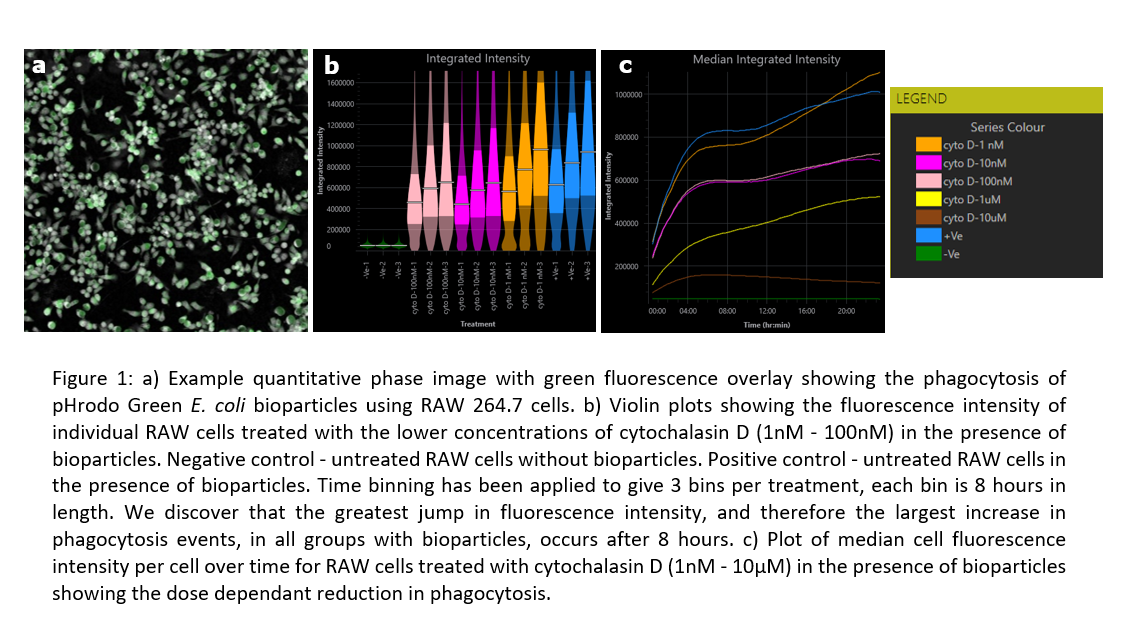
Through analysis of outputs generated by Livecyte’s Fluorescence Dashboard, a dose-dependent reduction in the median fluorescence intensity of the cells, therefore reduction in cell phagocytosis, with cytochalasin D treatment was determined. In addition, by analysing the cell fluorescence intensity and cell count, it was observed that the phagocytes were at their most active after 8 hours.
This data suggests cytochalasin adversely affects phagocytosis of bioparticles possibly though inhibiting the actin machinery needed by macrophages to internalize foreign particles. It also utilizes single-cell segmentation algorithms and time-lapse imaging to give a reliable reading into fluorescence intensity per cell and further insight into differences between phagocytic activity throughout the experiment without causing phototoxicity.
- References
- Uribe-Querol & Rosales, 2017. Control of Phagocytosis by Microbial Pathogens. Front Immunol. 8. 1368.
- Kapellos, Taylor, Lee, Cowley, James, Iqbal and Greaves, 2016. A novel real time Imaging platform to quantify macophage phagocytosis. Biochem. Pharmacol. 116. 107-119.
- Life Technologies, 2013. pHrodoTM Red and Green BioParticles® Conjugates for Phagocytosis. 1-6.
- Icha, Weber, Waters and Norden, 2017. Phototoxicity in live fluorescence microscopy, and how to avoid it. Bioessays. 39(8).
- Livecyte available from Phasefocus, UK.