Rapid (FLASH-FLIM) imaging of protoporphyrin IX in a tumour mimic in real time using a CMOS based widefield fluorescence lifetime imaging camera
- Abstract number
- 65
- Presentation Form
- Poster Flash Talk + Poster
- Corresponding Email
- [email protected]
- Session
- Stream 5: Imaging in Development and Disease
- Authors
- Graham Hungerford (1), Nathan Cumberbatch (1), Adam Holland (1), Kulwinder Sagoo (1)
- Affiliations
-
1. Horiba
- Keywords
FLIM, Fluorescence guided surgery, TCSPC, Tumour phantom
- Abstract text
A widefield fluorescence lifetime camera making use of time-correlated single-photon counting (TCSPC) was employed to image a tissue phantom in real time. The tissue phantom consisted of gellan gum and Intralipid with an inclusion doped with Protophyrin IX (PpIX) to provide a tumour mimic. The aim was to explore the use of fluorescence lifetimes as a potential tool to demarcate tumour boundaries in fluorescence guided surgery (FGS). The camera was CMOS based, with each pixel containing a single-photon avalanche diode (SPAD) and TCSPC timing electronics. This enabled simultaneous fluorescence lifetime acquisition in each of the pixels and the ability to present a fluorescence lifetime image in real time.
The identification of cancerous tissue can be subjective. The use of fluorescence, where a fluorophore can target a specific tissue and provide contrast with surrounding tissues has evolved, providing fluorescence guidance for surgical resection. Most instrumentation used in FGS relies on the intensity of the fluorescence emitted by the contrast agent, thus techniques are needed to quantify (ie standardise) the emission intensity. The fact that the fluorescence lifetime is an absolute measure, independent of fluorophore concentration, is advantageous as it can negate any calibration process. The most sensitive method to measure the fluorescence lifetime is that of TCSPC and recent developments in CMOS based technology, principally SPADs and photon timing electronics, have enabled sensors consisting of detector arrays with associated timing electronics to be fabricated [1]. A widefield approach minimises the number of moving parts (no scan module) and means that a parallel approach to data collection can be employed, with the ability to rapidly acquire data.
The fluorescence from protoporphyrin IX (PpIX) has been employed to characterise cellular activity and assist in the visualisation of tumour cells. Its formation can be induced by 5-aminolevulonic acid (5-ALA) which is metabolised by tumour cells to form PpIX, where it is localised within the cells. Here PpIX was included in a construct consisting of lipid mixture, Intralipid [2] (employed to simulate fat content and optical scattering), in a gellan gum matrix and PpIX incorporated in intralipid in aqueous solution. The samples are imaged using commercial widefield TCSPC camera (Horiba Scientific FlimeraTM) based on a sensor chip with 192 x 128 pixels [3]. Each pixel contains both detection and photon timing enabling the Fluorescence Lifetime Acquisition by Simultaneous Histogramming (FLASH). This “FLASH-FLIM” approach enables widefield fluorescence lifetime images in real time to be acquired [4].
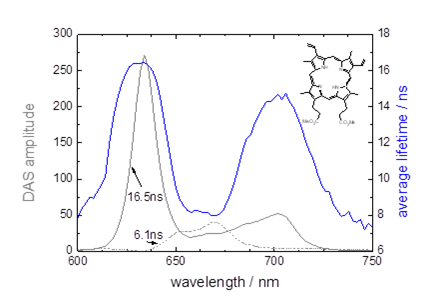
The emission of PpIX can be affected by the presence of photoproducts and aggregates [5] which can influence the monitored lifetime of the emission, especially if viewed using a longpass filter. Figure 1, measured using a Horiba Scientific DeltaFlex system, shows decay associated spectra (DAS) for PpIX in Intralipid. The presence of two emitting species was determined and means that the average lifetime will vary with emission wavelength; dependent on the relative quantities of the two emissions. Also the observed lifetime is therefore expected to be shorter than that of the monomeric form (16.5ns). Considering the whole wavelength range shown the average lifetime is close to 11 ± 3ns.
Figure 1. Decay associated spectra and the average lifetime for PpIX in Intralipid.
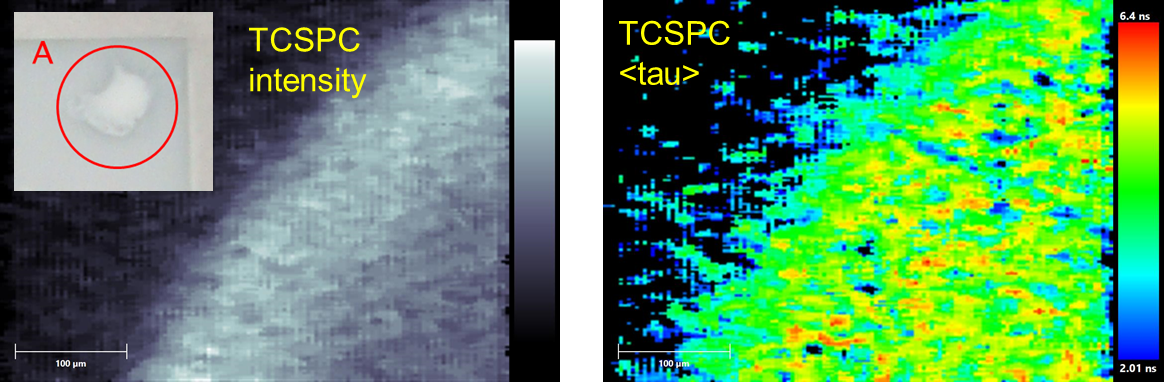
Examining the margin of part of a PpIX labelled inclusion (region A, insert in figure 2) using the widefield FLIM camera showed that it was possible to acquire an image in a short period of time (1 second), with sufficient photons to obtain a lifetime image. As little as 200 photons events can yield lifetime data [6], however the accuracy of the lifetime determination will deteriorate with few counts and the lifetime obtained is not as large as expected. However if the purpose is to use it as a tool by which to gain contrast then its absolute value may not be of critical importance.
Figure 2. Intensity and average lifetime lifetime images of part of region A (inset) of a PpIX labelled inclusion within a gellan / Intralipid construct.
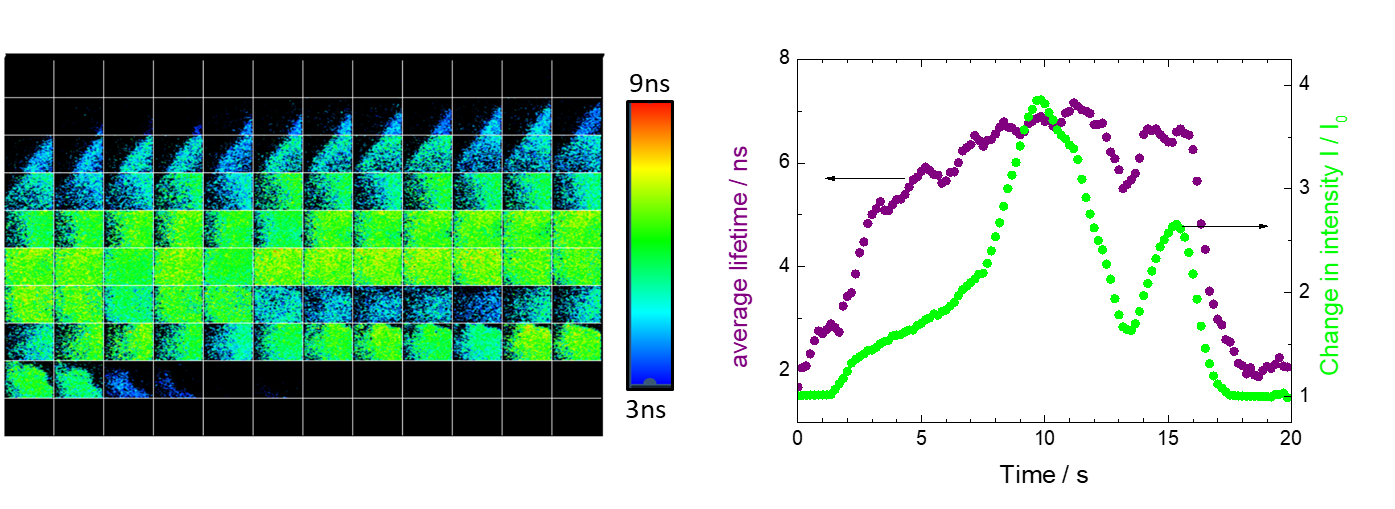
If the use of the fluorescence lifetime parameter is to be used as a tool in FGS then the ability to visualise, at a rate that would enable real time feedback to a surgeon is of importance. To assess this the sample was moved on the stage under the FLIM camera for a period of 20 seconds and the data acquired as the inclusion boundaries were traversed. A montage of the images at a rate of 6 frames per second. This rate would give sufficient real time feedback and enable sufficient photons for the lifetime parameter to be used as a tool in this instance. The outcome is shown in figure 3.
Figure 3. Montage, left, (6 fps, images left to right) of a 20 second measurement moving the tissue construct under the FLIM camera so that the boundaries of the PpIX inclusion are traversed. Right comparison of change in intensity and average lifetime from each frame.
The analysis of the frames, in terms of average lifetime and change in intensity shows that a greater change towards the boundaries of the inclusion are observed using the fluorescence lifetime parameter as compared to the intensity measure (figure 3). Thus showing the potential of its usage in FGS applications.
The acquisition speed using the parallel FLASH-FLIM approach shows the potential of using the fluorescence lifetime as a parameter that can distinguish the tumour margin, while giving real time feedback, in the area of fluorescence guided surgery.
- References
[1] C. Bruschini et al., 2019. Light Sci. Appl. 8, 87.
[2] M.Lepore and I. Del, 2019. Open Biol. J., 13, 163-172.
[3] R.K. Henderson et al., 2019. IEEE J. Solid-State Circuits. 54, 1907-1916.
[4] K. Sagoo et al., 2021. Methods Appl. Fluoresc. 9, 015002.
[5] L. Alston et al., 2018. J. Biomed. Opt., 23, 097002.
[6] D. McLoskey et al., 2011. Meas. Sci. Technol. 22, 067001.