Understanding Cu-Alumina interactions in redox conditions for Chemical Looping Combustion (CLC) application – A multi-scale correlative electron and X-ray microscopy study
- Abstract number
- 92
- Presentation Form
- Submitted Talk
- DOI
- 10.22443/rms.mmc2021.92
- Corresponding Email
- [email protected]
- Session
- Stream 1: EMAG - Energy and Energy Storage Materials
- Authors
- Sharmin Sharna (1, 2), Virgile Rouchon (1), Anne-Lise Taleb (1), Christèle legens (1), Stefan Stanescu (3), Arnold Lambert (1), Anne-Sophie Gay (1), David Chiche (1), Ovidiu Ersen (2)
- Affiliations
-
1. IFP Energies nouvelles
2. Institut de Physique et de Chimie des Matériaux de Strasbourg
3. Synchrotron Soleil
- Keywords
Chemical Looping Combustion, CLC, Oxygen Carrier, Copper Oxide, Copper, CuO-Alumina, Oxidation-Reduction, in-situ STEM, STXM-XAS, Correlative microscopy
- Abstract text
Chemical Looping Combustion (CLC) is a midterm solution for fossil fuel utilization with inherent carbon dioxide capture, based on the use of an oxygen carrier material. The oxygen carriers (OC) replace air to provide oxygen to a wide range of fuels for combustion, via reduction/oxidation cycles in a circulating fluidized bed reactor at high temperature [1]. Copper oxide supported on alumina grain (CuO/Al2O3) has been widely considered as a promising oxygen carrier (OC) for industrial use in CLC, due to its benign nature and flexible redox behavior that ensures high reactivity and oxygen transfer capacity. However, the OC sustains successive high temperature (800-900°C) reduction (combustion) and oxidation (regeneration of oxide phase) reaction cycles which lead to chemical and morphological changes in the material causing the degradation in the oxygen carrying properties. The evolution in the cycled material is attributed to the diffusion of the Cu-based phases at the grain scale [2].
Herein, we are bridging the gap in understanding between the observed μm-scale migration of Cu-based phases and nanoscale transformations of the Cu nanoparticles (NPs) by employing a multi-scale characterization approach using Scanning Transmission X-ray (STXM) and Scanning Transmission Electron (STEM) Microscopies, respectively. Correlative spectro-microscopy is used to characterize the evolution of interacting Cu and Alumina phases after different aging times under successive redox cycles. Furthermore, in-situ STEM, mimicking redox cycling, is used to achieve a nanoscale view of the phase/morphological evolution of the Cu NPs and to identify the sintering mechanism of the copper phases during successive reduction and oxidation steps.
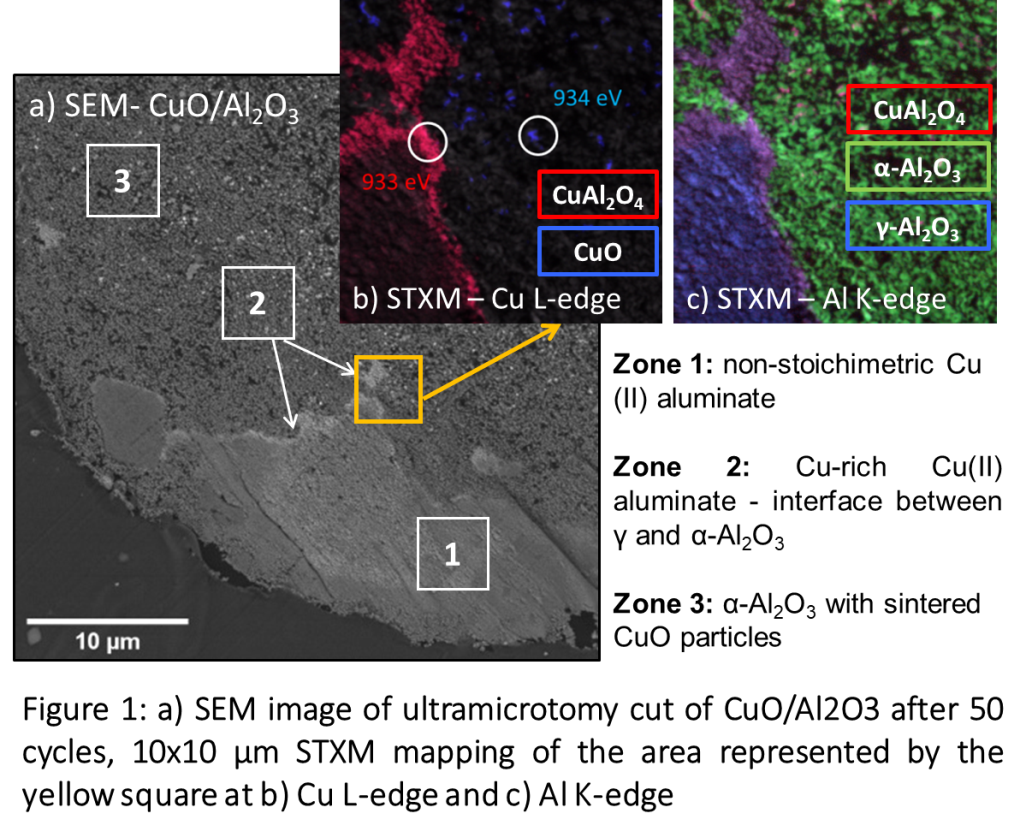
We have studied CuO phase supported on 50-100 µm sized γ-Al2O3 grain, produced via incipient wetness impregnation of 13%wt CuO and calcined at 800 °C. To mimick the CLC cycling, the fresh samples were subjected to oxidation and reduction under air and H2 at 900 °C, in a thermogravimetric analyser (TGA). Ultramicrotomy sections of 100 nm thickness of were prepared for SEM, STXM and TEM characterizations. STXM was performed at the HERMES beamline of the Soleil Synchrotron facility. Energy stacks and mappings were performed at the Cu L-edge and Al K-edge in order to identify the specific spectral features of each compound and to map their distribution with ~40 nm resolution. Using external standards of CuO, Cu(II) aluminate (CuAl2O4), γ and α-Al2O3, spectral features at 934 eV, 933 eV, 1579 eV and 1581 eV were representative of each phase respectively. In-situ TEM was performed using a probe Cs-corrected microscope equipped with Protochips’ ”Atmosphere” in-situ gas setup with sealed environmental cell (E-Cell), operating at atmospheric pressure.
The fresh grains are composed of γ -Al2O3 with homogeneously dispersed CuO nanoparticles (10-20 nm) and large (µm sized) CuO particles on the outskirts of the grain. With progressive cycling, at the grain scale (µm), we observe the disappearance of the large CuO particles and the propagation of a gamma to alpha reaction front in the solids (Figure 1). At smaller scale (10 x 10 µm2), this front displays a well-defined structural-chemical gradient characterized by (figure 2b): zone 1, non-stoichiometric Cu (II) aluminate; zone 2, an intermediate thin layer (< 200 nm) of Cu (II) aluminate, enriched in Cu compared to zone 1; zone 3, α-Al2O3 phase containing large CuO particles. The proportion of copper shows a strong variation from 10 wt% Cu in zone 1, to ~25wt% at the edge of the reaction front in zone 2. In zone 3, copper is concentrated in localized particles of several tens of nm in diameter indicating a strong sintering effect.
In-situ STEM observation at 900 °C under H2-reduction have shown the migration of copper to form copper nanoparticles from a starting oxidized sample mainly composed of homogeneous Cu (II) aluminate (Figure 2). It is suspected that the expelling of Cu from the spinel structure of Cu (II) aluminate favors the formation of the corundum structure of Cu-free Al2O3. This suggests that the mobility of copper during redox cycling is linked to the phase transition of γ- to α-Al2O3. However, the γ- to α-Al2O3 transition is not favored in the pure Al-O system below 1000 - 1100 °C [3]. Reversely, the oxidative formation of spinel is only possible in the CuO+γ-Al2O3 system and thermodynamically limited in the case of CuO+α-Al2O3, at 900 °C [4]. To minimize the energy of the system, the mobility of copper on alumina during oxidation is sufficient to either form (1) CuAl2O4 at the expense of g-Al2O3, and (2) larger CuO particles on α-Al2O3 by sintering. Through a step by step migration mechanism induced by the redox cycling, copper is being gradually concentrated at the external border of zone 3, the latter expanding through the grains until reaching complete a-Al2O3 formation. Fractions of copper not being able to reach γ-Al2O3 particles during an oxidation cycle sinter to form larger, more stable particles on the α-Al2O3 surface.
This study demonstrates how correlative multi-scale imaging techniques can be employed to reveal the dynamic interactions of metal/metal oxides and ceramic supports in complex reactive systems.- References
[1] Adanez et al., Progress in Energy and Combustion Science, 38, (2012), 215-282
[2] Lambert et al., Fuel, 216 (2018), 71–82
[3] Boumaza et al., Journal of Solid State Chemistry, 182 (2009) 1171–1176
[4] Hu et al., RSC Advance, 6 (2016),113016–113024